- Department of Intensive Care Medicine, Manipal Hospital Bengaluru, Bengaluru, Karnataka, India.
- Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Haematology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Shalvi Mahajan, Department of Anesthesia and Intensive Care, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_296_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gopala K. Ravi1, Nidhi Panda2, Jasmina Ahluwalia3, Rajeev Chauhan2, Navneet Singla4, Shalvi Mahajan2. Effect of tranexamic acid on blood loss, coagulation profile, and quality of surgical field in intracranial meningioma resection: A prospective randomized, double-blind, placebo-controlled study. 07-Jun-2021;12:272
How to cite this URL: Gopala K. Ravi1, Nidhi Panda2, Jasmina Ahluwalia3, Rajeev Chauhan2, Navneet Singla4, Shalvi Mahajan2. Effect of tranexamic acid on blood loss, coagulation profile, and quality of surgical field in intracranial meningioma resection: A prospective randomized, double-blind, placebo-controlled study. 07-Jun-2021;12:272. Available from: https://surgicalneurologyint.com/surgicalint-articles/10854/
Abstract
Background: Resection of intracranial meningioma has been associated with significant blood loss. Providing a clear surgical field and maintaining hemodynamic stability are the major goals of anesthesia during meningioma surgery. Tranexamic acid has been used to reduce blood loss in various neurosurgical settings with limited evidence in literature. A randomized, double-blind, and placebo-controlled trial was conducted to evaluate the efficacy of tranexamic acid on blood loss, coagulation profile, and quality of surgical field during resection of intracranial meningioma.
Methods: Thirty patients aged 18–65 years undergoing elective meningioma resection surgery were given either tranexamic acid or placebo (0.9% saline), tranexamic acid at a loading dose of 20 mg/kg, and infusion of 1 mg/kg/h during surgery. The intraoperative blood loss, coagulation profile, and the surgical field using Likert scale were assessed.
Results: The patients in tranexamic group had significantly decreased intraoperative blood loss compared to the placebo group (616.42 ± 393.42 ml vs. 1150.02 ± 416.1 ml) (P = 0.02). The quality of the surgical field was better in the tranexamic group (median score 4 vs. 2 on Likert Scale) (P P = 0.047).
Conclusion: Tranexamic acid bolus followed by infusion reduces perioperative blood loss by 46.43% and blood transfusion requirement with improved surgical field and coagulation profile in patients undergoing intracranial meningioma resection surgery.
Keywords: Blood loss, Coagulation profile, Meningioma surgery, Tranexamic acid
INTRODUCTION
Intracranial meningiomas account for 13–40% of primary brain tumors.[
Tranexamic acid (trans-4-aminomethyl-cyclohexane-1-carboxylic acid) is a synthetic lysine analog which acts by competitively blocking the lysine-binding residues on fibrin clot and prevents the conversion of plasminogen to plasmin, thus inhibiting clot lysis and acts as antifibrinolytic agents. It has been used to reduce blood loss during coronary revascularization, liver resection, and obstetric and orthopedic procedures. Studies have shown that it decreases intraoperative blood loss by up to 40%.[
The aim of this trial was to assess the efficacy of tranexamic acid on bleeding in the intraoperative and postoperative period as primary outcome, while the secondary outcomes were the quality of the surgical field, requirement of blood transfusion, and the coagulation profile of the patient preoperatively, intraoperatively and postoperatively during intracranial meningioma surgery.
MATERIALS AND METHODS
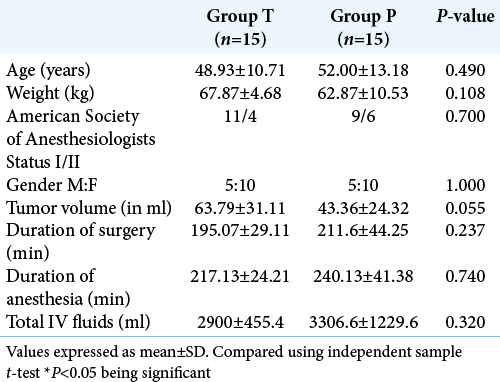
A prospective, randomized, and double-blind controlled trial was carried out after approval from Institutional Ethics Committee and written informed consent from patient or relative. Thirty American Society of Anesthesiologists’ I and II patients, aged between 18 and 65 years, undergoing elective surgery for excision of meningiomas were included in the study. These meningiomas were more than 5 cm in size (in largest of dimension). Patients with previous thromboembolic events, coagulopathy or on anticoagulation therapy, impaired renal function (Creatinine >1.1 mg/dl), abnormal liver function, or allergic to tranexamic acid were excluded from the study. Patients who received any kind of pretreatment embolization were not included in the study.
Randomization and blinding
Patients were randomized using a computer generated 1:1 allocation ratio random table into two groups. The results were kept in sealed envelopes. Patients were allocated randomly into Group T and Group P. Patients in Group T received a bolus dose of 20 mg/kg of tranexamic acid (Cyclokapron, Pfizer, New York) before starting the surgery followed by an infusion at a rate 1 mg/kg/h till the end of the surgery and patients in Group P received the corresponding volume of normal saline as placebo for blinding of the study drug. The anesthesiologist who prepared the study drug according to the random number assigned did not participate in anesthetizing the patient or collecting the data. The anesthesiologist who collected the data and the operating surgeon was blinded to the study drug.
Anesthesia protocol
A standard anesthesia protocol was followed in all the patients. The patients were induced with propofol 1–2 mg/kg and trachea were intubated with vecuronium 0.1 mg/kg. Anesthesia was maintained by propofol infusion 75–150 μg kg/h, nitrous oxide-oxygen mixture (50:50) with 2L of fresh gas flow, and intermittent doses of vecuronium 0.02 mg/kg. Intraoperative analgesia was maintained with fentanyl 2 μg/kg bolus followed by 1 μg/kg/h infusionand ventilation was maintained keeping PaCO2 of 35–38 mm Hg. All patients both in intervention and control arm received prophylactic anticonvulsant (Inj. Phenytoin 15 mg/kg IV) after anesthetic induction as an institute protocol.
An arterial line and a central venous line were secured. A heparinized arterial blood sample was sent before starting of surgery to know “Baseline hematocrit.” Quality of surgical field was assessed by a single surgeon (NS) who operated all the cases and evaluated the quality of the field of surgery using a 5-point Likert scale where scoring was done as excellent=5, very good=4, good=3, satisfactory=2, and bad=1. The surgeon was blinded to the group patient was allocated and test drug received.
Coagulation profile of all patients was assessed using five tests, namely prothrombin time (PT), activated partial thromboplastin time (aPTT), D-dimer level, fibrinogen level, and tissue plasminogen activator level preoperatively, intraoperatively (90 min after start of surgery), and postoperatively (24 h after surgery). The PT, aPTT, fibrinogen, and D-dimer levels were analyzed using STA Compact Coagulation Analyzer (STASD, France). Fibrinogen and D-dimer levels were measured using Claus method and Latex Immunoassay, respectively. The tissue plasminogen activator level was assessed using a commercial enzyme-linked immunosorbent assay kit (Assaypro, Missouri, USA).
The infusion of tranexamic acid was stopped at the end of the surgery just before skin closure. Arterial blood sample was evaluated for “Final hematocrit” estimation at the end of skin closure. The hematocrit was analyzed using Siemens Bayer 248 Blood Gas Analysis machine (Siemens, Minnesota). Blood was transfused if the intraoperative hematocrit was ≤27%.
Postoperatively, the patients were assessed for the volume of blood collected in the closed suction drain in 24 h postsurgery and blood sample was collected for postoperative coagulation profile of the patient.
The blood loss was calculated using Modified Gross formula and if there was intraoperative blood transfusion then, formula by Brecher et al. was used.[
Using Modified Gross Formula → Blood Loss = EBVx [Hct (i) - Hct (f)]/Hct (m)
where EBV was Estimated Blood Volume {Body Weight in kgs × (70–75) ml/kg} (where 70 ml/kg-females, 75 ml/ kg- male), Hct (i) is initial hematocrit, Hct (m) is mean hematocrit and Hct (f) is final hematocrit.
Using formula by Brecher et al. (when patient received intraoperative blood transfusion)[
Blood loss = EBV × ln [Hct (i)/Hct (min)] (A)
where EBV was estimated blood volume, Hct (i) was the hematocrit at the start of surgery and Hct (min) was the minimal Hct (≤27%).
Blood Loss = (RBCT–RBCF)/Hct(min) (B)
where RBCT (total amount of red blood cell transfused) = No. of units of PRBC transfused x volume of each PRBC × hematocrit of PRBC transfused. The hematocrit of the PRBC was taken as 60% as per blood bank standards, while the volume was mentioned on individual units.
RBCF = Hematocrit at the end of surgery
RBCF = (Hematocrit at the end of surgery-Minimum hematocrit) × EBV
Hct (min) = transfusion trigger kept as 27%.
Total blood loss = (A) ± (B).
Statistical analysis
The statistical analysis was performed using Statistical Package for the Social Sciences Inc., Chicago, IL, version 21. The discrete categorical data were presented as n (%); continuous data were analyzed by the mean and standard deviation or median and interquartile range (IQR). Demographic variables such as age, weight, duration of surgery, and anesthesia were presented as mean ± standard deviation and were compared among the two groups using one-way analysis of variance. Categorical variables such as sex and quality of surgical field were presented as frequency or percentage and were compared among the two groups using Chi-square/Fisher’s exact test. The Kolmogorov–Smirnov test was applied to assess the normality of the measured data such as blood loss. In normally distributed data, Student’s t-test was used and in skewed data, Mann–Whitney U test was applied to compare between the two groups. P < 0.05 was considered as significant.
Power analysis
Sample size was estimated after conducting a pilot study in 10 patients undergoing surgery for intracranial meningioma (>5 cm). Intraoperative mean blood loss was estimated to be 1000 ml with a standard deviation of ± 450 ml. To detect a 50% change in blood loss, the sample size calculated to 15 per group at a power of 80% and confidence interval of 95%. Therefore, 30 patients were included in this study and randomly divided equally into two groups, placebo group (Group P) and study group (Group T).
RESULTS
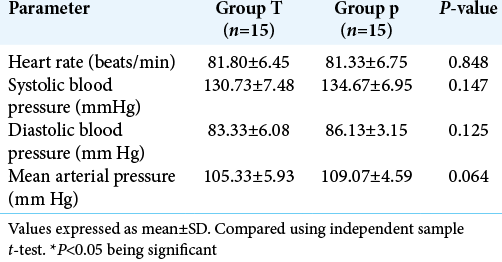
Patients in both the groups (n = 15/group) were enrolled into the study and followed up in perioperative period for the coagulation profile and volume of blood loss in closed drain 24 h after surgery [
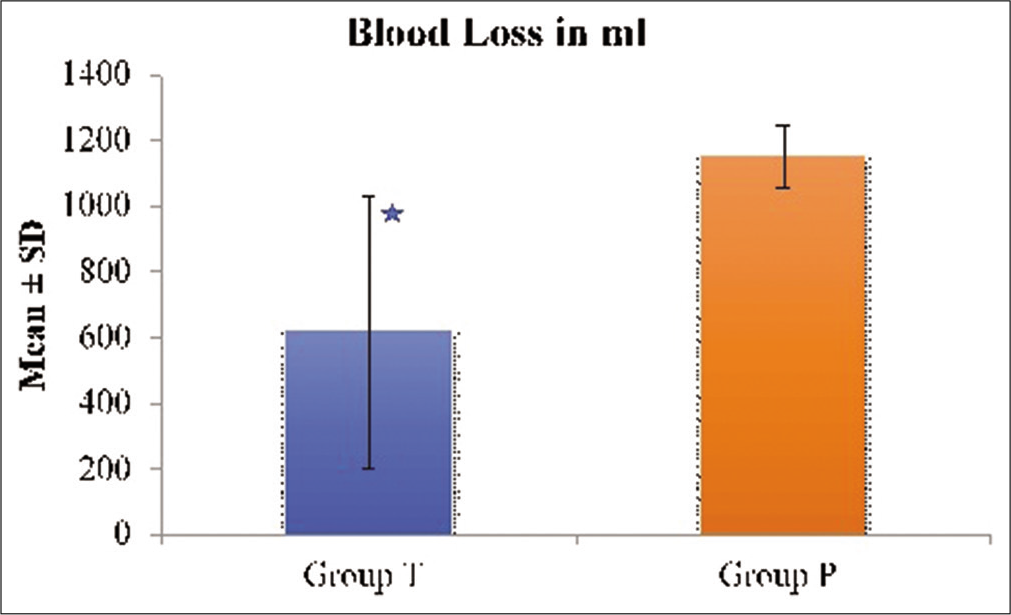
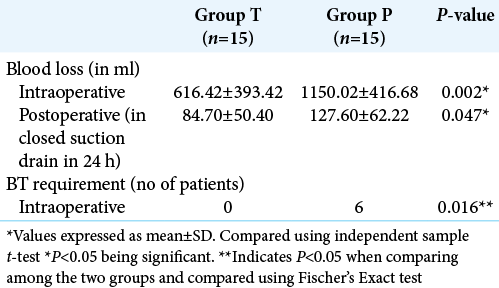
There was a statistically significant decrease in blood loss in Group T (616.4 ± 393.4 ml vs. 1150 ± 416.1 ml, P = 0.002). This equated to 46.43% blood loss reduction in Group T compared to Group P [
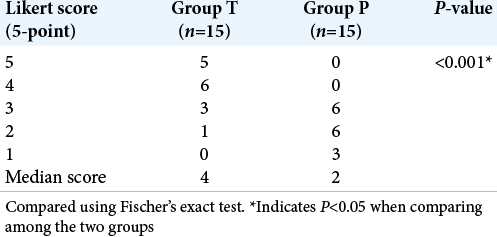
The quality of the surgical field was graded by a blinded, single neurosurgeon who operated all the cases using a 5-point Likert scale. The median score in Group T was 4 (good) (IQR 3–5), while in Group P, it was 2 (satisfactory) (IQR 2–3) [
The number of patients requiring blood transfusion was six and all these patients belonged to group P and none of the patients in Group T required blood transfusion (P = 0.016) [
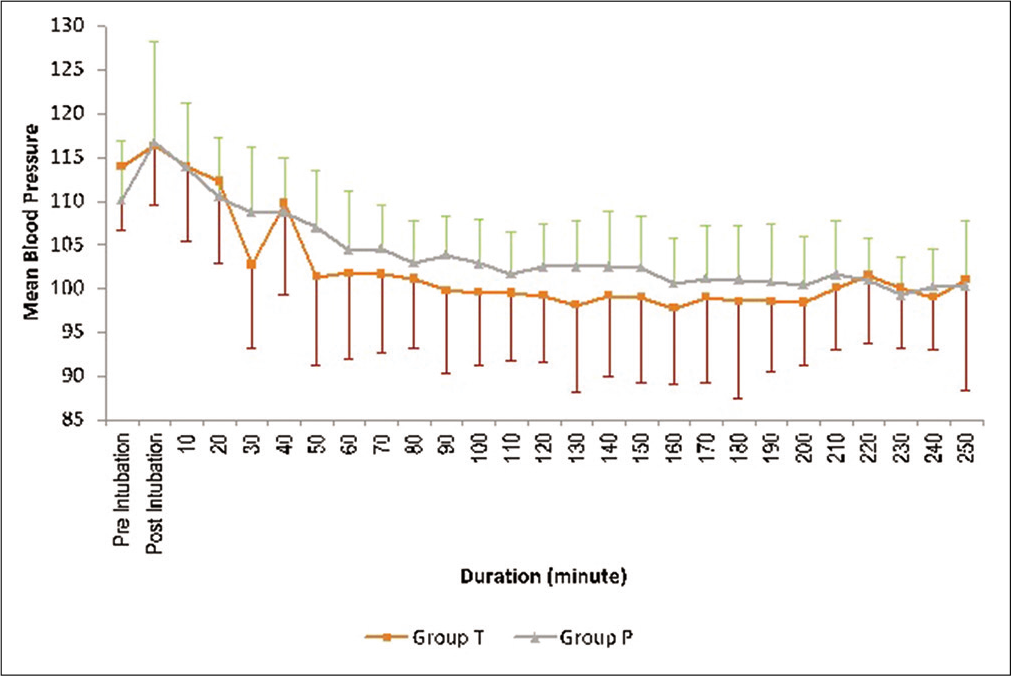
Patients in the placebo arm suffered significant blood loss compared to the treatment group but no differences in intraoperative parameters were seen due to higher fluid administration (colloid and crystalloid) and significantly higher packed red blood cells transfusion as depicted in [
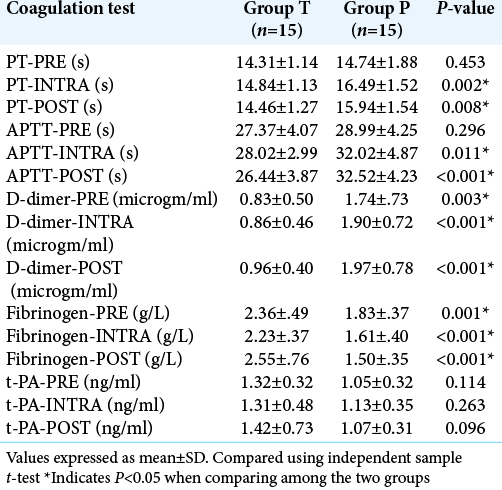
The coagulation tests were compared among the two groups using t-test for equality of means. The values of PT and aPTT were longer intraoperatively and postoperatively in Group P compared to Group T and it was statistically significant. The D-dimer and fibrinogen levels were elevated preoperative, intraoperative, and postoperatively in Group P compared to Group T and were statistically significant [
None of the patients in either group had seizures, thromboembolic episode, or any other major complication in the intraoperative and 24 h postoperative period.
DISCUSSION
This study showed a significant decrease in intraoperative blood loss and 24 h collection of blood in a closed drain in patients receiving intraoperative Tranexamic acid bolus and infusion in patients undergoing intracranial meningioma excision. Surgical field and perioperative coagulation profile were also found to be better in the same group.
Intracranial meningioma excision surgeries are one of the commonly performed procedures in major neurosurgical centers around the world. In this surgery, increased vascularity of tumor, increased tumor size, and prolonged operation time increase the risk of major intraoperative bleeding.[
Tranexamic acid was used in our study in a loading dose of 20 mg/kg followed by infusion of 1 mg/kg/h throughout intraoperative period. We observed a significant difference (P = 0.002) in the blood loss among the two groups. The mean blood loss in the tranexamic acid group was 616.23 ± 393.41 ml and in the placebo group, it was 1150.28 ± 474.45 ml. There was an absolute reduction in blood loss by 46.43% in this study when tranexamic acid was used. All the patients were followed up for 24 h and observed that blood collected in closed suction drain which was significantly less in the tranexamic acid group as compared to the placebo group (84.7 ± 50.4 ml vs. 127.6 ± 62.2 ml, P < 0.05). None of the patients in tranexamic acid group and six patients in the placebo group required intraoperative blood transfusion and the difference was statistically significant (P = 0.016). Similar to our result Hooda et al. observed a significant decrease in intraoperative blood loss in patients who received intraoperative tranexamic acid in the same dose, but they did not observe any difference in blood transfusion requirement between the groups.[
In our study, the surgical field was evaluated by a blinded surgeon who operated all the cases using 5 points Likert scale. We observed surgical field was better in the tranexamic acid group (median score 4) (good surgical field) compared to placebo group (median score 2) (satisfactory surgical field) (P < 0.001). The coagulation profile of our patients was assessed preoperative, intraoperative, and postoperatively. The PT was within the normal limits in both the groups preoperatively, but they were found to be significantly prolonged in the placebo group intraoperatively and postoperatively (P < 0.001) as compared to tranexamic group. The intraoperatively and postoperative PT in patient receiving tranexamic acid were better than those who did not receive Tranexamic acid (P = 0.002, P = 0.008, respectively). This difference can be explained because of the fact that brain tissue is rich in tissue factor and these factors are released during tissue dissection in brain.[
The fibrinogen levels were within the normal limits in the tranexamic group while it is decreased significantly in the placebo group in preoperatively, intraoperatively, and postoperatively (P < 0.001) period. The fibrinolysis due to release of tissue plasminogen activator and tissue factor leads to increased conversion of fibrinogen to fibrin and hence decreasing the fibrinogen levels.[
The D-dimer levels were lower in the tranexamic acid group compared to the placebo group preoperative, intraoperative, and postoperatively and they were statistically significant (P < 0.05). The D-dimer levels were raised in both the groups preoperatively (>0.5 μg/L). Hence, indicating preoperative fibrinolysis but the values increased intraoperatively and postoperatively in the placebo group not in tranexamic acid group due to fibrin stabilizing action of the drug (tranexamic acid) by preventing the conversion of plasminogen to plasmin.[
The decreased fibrinogen level and increased D-dimer levels in the placebo group preoperatively, which was statistically different compared to tranexamic acid group, could be a reason for increased blood loss and is a limitation of our study.
The tissue plasminogen activators were within the normal range in both the groups throughout surgery and they were not statistically significant. Hence, from the above coagulation tests performed in our patients among the tranexamic acid and placebo groups, we observed that patients with intracranial meningioma are in a fibrinolytic state leading to deranged coagulation tests perioperatively and this can be corrected by the use of anti-fibrinolytic drugs like tranexamic acid which can correct the hyper-fibrinolytic state, thereby reducing blood loss and decreasing the need for intraoperative blood transfusion. We have not observed any thromboembolic events, postoperative seizures, or any major complications in all patients included in the study till 24 h postoperatively. Our result about safety of tranexamic acid is supported by the meta-analysis done by Yokobori et al. They assessed 5076 patients who had received tranexamic acid from seven studies and observed that thromboembolic events were not more than that of the control group.[
The dose of tranexamic acid varies based on indications of its use. In cardiac surgeries, a recent review showed that maximum dose of 30 mg/kg can be used and it was found to be safe without risk of complications like seizures.[
Another landmark study on use of tranexamic acid is CRASH-3 trial, which concluded that its use is beneficial in early (<3 h) mild to moderate traumatic brain injury with mortality benefit (18% vs. 19.5%, P = 0.03) and decreased risk of death (RR 0•78 [95% CI 0•64–0•95]) and no difference in adverse outcomes including incidence of vaso-occlusive events and seizures.[
Ageron et al. observed that tranexamic acid has been shown to significantly increase overall survival from bleeding with no heterogeneity by site of bleeding. However, treatment delay reduced the treatment benefit and early treatment with tranexamic acid improved survival by more than 70%.[
The strength of our study includes evaluation of coagulation profile in patients undergoing meningioma excision receiving tranexamic acid which no previous published study had done. The second point of strength of this study is that surgical field assessment was done by a single-blinded neurosurgeon in all the cases. It provides uniformity in the assessment of subjective criteria.
Limitations
Limitations of the study include small size of the study population which is though sufficient to check decrease in blood loss but not adequate to check any postoperative complications. The histology of the tumor and extent of tumor resection was not noted individually in all the cases which could have added strength to our conclusion of the efficacy of tranexamic acid in meningioma surgery.
CONCLUSION
Tranexamic acid administration (bolus followed by intraoperative infusion) reduces intraoperative blood loss in meningioma resection surgeries by 46.43% and decreases the need for intraoperative packed cell transfusion and improves the quality of surgical field. It also decreases the 24 h closed suction drain output and improves the coagulation parameters. No thromboembolic complications within 24 h were observed in any of the patient received tranexamic acid. A large population study is necessary to firmly establish the efficacy and complications of tranexamic acid in patients undergoing intracranial meningioma.
List of drugs used
Propofol Fentanyl Vecuronium Tranexamic acid Phenytoin.
Ethics approval and consent to participate
Clearance to conduct the study was obtained from the Institute Ethics Committee in accordance with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from either patients or next of the kin all patients who participated in the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Brecher ME, Monk T, Goodnough LT. A standardized method for calculating blood loss. Transfusion. 1997. 37: 1070-4
2. Roberts I, Shakur H, Afolabi A, Brohi K, Coats T. The importance of early treatment with Tranexamic acid in bleeding trauma patients: An exploratory analysis of the CRASH-2 randomized controlled trial. Lancet. 2011. 377: 1096-101
3. Shakur H, Roberts I, Bautista R, Caballero J, Coats T. Effects of Tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant hemorrhage (CRASH-2): A randomized, placebo-controlled trial. Lancet. 2010. 376: 23-32
4. 5. Gayet-Ageron A, Prieto-Merino D, Ker K, Shakur H, Ageron FX, Roberts I. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: A meta-analysis of individual patient-level data from 40138 bleeding patients. Lancet. 2018. 391: 125-32 6. Goh KY, Tsoi WC, Feng CS, Wickham N, Poon WS. Haemostatic changes during surgery for primary brain tumours. J Neurol Neurosurg Psychiatry. 1997. 63: 334-8 7. Gross JB. Estimating allowable blood loss: Corrected for dilution. Anesthesiology. 1983. 58: 277-80 8. Hodgson S, Larvin JT, Dearman C. What dose of tranexamic acid is most effective and safe for adult patients undergoing cardiac surgery?. Interact Cardiovasc Thorac Surg. 2015. 21: 384-8 9. Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017. 41: 132-8 10. Hooda B, Muthuchellappan R. Tranexamic acid in neuroanesthesia and neurocritical care: Time for its critical appraisal. J Neuroanaesthesiol Crit Care. 2019. 6: 257-66 11. Hsu SY, Huang YH. Characterization and prognostic implications of significant blood loss during intracranial meningioma surgery. Transl Cancer Res. 2016. 5: 797-804 12. Hu W, Xin Y, Chen X, Song Z, He Z, Zhao Y. Tranexamic acid in cerebral hemorrhage: A meta-analysis and systematic review. CNS Drugs. 2019. 33: 327-36 13. McCormack PL. Tranexamic acid a review of its use in treatment of hyperfibrinolysis. Drugs. 2012. 72: 585-617 14. McDermott MW, Wilson CB, Youmans W, Julian R, Bullock RM, Burcheli KJ.editors. Meningioma. Neurological Surgery: A Comprehensive Guide to the Diagnosis and Management of Neurosurgical Problems. New York: Saunders; 1996. p. 2782-24 15. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch Surg. 2012. 147: 113-9 16. Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth. 2013. 111: 549-63 17. Ricky HW, Wong AK, Vick N, Farhat HI. Natural history of multiple meningiomas. Surg Neurol Int. 2013. 4: 71 18. Sawaya R, Cummins CJ, Kornblith PL. Brain tumors and plasmin inhibitors. Neurosurgery. 1984. 151: 795-800 19. Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M. Tranexamic acid to improve functional status in adults with spontaneous intracerebral haemorrhage: The TICH-2 RCT. Health Technol Assess. 2019. 23: 1-48 20. Tsementzis SA, Hitchcock ER, Meyer CH. Benefits and risks of antifibrinolytic therapy in the management of ruptured intracranial aneurysms. A double-blind placebo-controlled study. Acta Neurochir (Wien). 1990. 102: 1-10 21. Tsementzis SA, Meyer CH, Hitchcock ER. Cerebral blood flow in patients with a subarachnoid haemorrhage during treatment with tranexamic acid. Neurochirurgia. 1992. 35: 74-8 22. Tsuda H, Oka K, Noutsuka Y, Sueishi K. Tissue-type plasminogen activator in patients with intracranial meningiomas. Thromb Haemost. 1988. 60: 508-13 23. Vel R, Udupi BP, Prakash MV, Adinarayanan S, Mishra S, Babu L. Effect of low dose tranexamic acid on intra-operative blood loss in neurosurgical patients. Saudi J Anaesth. 2015. 9: 42-8 24. Vermeulen M, Lindsay KW, Murray GD, Cheah F, Hijdra A, Muizelaar JP. Antifibrinolytic treatment in subarachnoid hemorrhage. N Engl J Med. 1984. 311: 432-7 25. Yokobori S, Yatabe T, Kondo Y, Kinoshita K.editors. Japan Resuscitation Council (JRC) Neuroresuscitation Task Force and the Guidelines Editorial Committee.Efficacy and safety of tranexamic acid administration in traumatic brain injury patients: A systematic review and meta-analysis. J Intensive Care. 2020. 8: 46