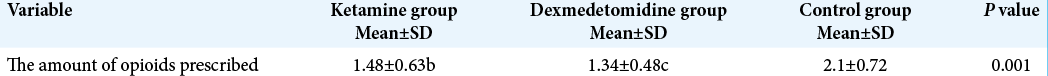- Department of Anesthesiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
DOI:10.25259/SNI_850_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nasim Nikoubakht, Mahzad Alimian, Seyed Hamid Reza Faiz, Pooya Derakhshan, Mohammad Saleh Sadri. Effects of ketamine versus dexmedetomidine maintenance infusion in posterior spinal fusion surgery on acute postoperative pain. 26-Apr-2021;12:192
How to cite this URL: Nasim Nikoubakht, Mahzad Alimian, Seyed Hamid Reza Faiz, Pooya Derakhshan, Mohammad Saleh Sadri. Effects of ketamine versus dexmedetomidine maintenance infusion in posterior spinal fusion surgery on acute postoperative pain. 26-Apr-2021;12:192. Available from: https://surgicalneurologyint.com/surgicalint-articles/10751/
Abstract
Background: One of the most challenging issues after posterior spinal fusion (PSF) surgery is providing appropriate pain control measures to enhance recovery of the patients. We aimed to compare effects of ketamine versus dexmedetomidine infusion during maintenance of anesthesia on acute postoperative pain in PSF surgery.
Methods: In a double-blinded randomized clinical trial, 87 patients candidates for PSF surgery were randomly assigned into three groups. Anesthesia protocol for all groups was the same except: the first group received 0.2 mcg/kg/h dexmedetomidine infusion, the second received 0.1 mg/kg/h ketamine infusion, and control group received normal saline infusion as a placebo. Pain intensity by VAS scale and level of sedation by Ramsey scale were assessed, and amount of opioid prescribed after surgery was measured and compared for patients during the recovery and at 2, 4, 6, 12, and 24 h after surgery in three groups, and hypotension and bradycardia during operation were reported.
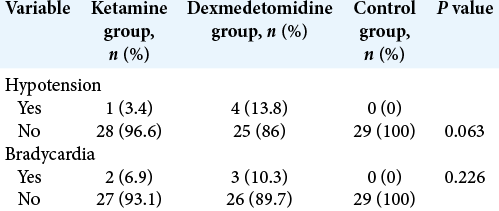
Results: There was a significant difference among the groups regarding pain intensity and amount of opioids during recovery and at 2, 4, 6, 12, and 24 h after surgery. Pain intensity and amount of opioids for ketamine and dexmedetomidine groups were significantly lower than those in the controls during recovery and at the hours after surgery. There was no significant difference regarding bradycardia and hypotension and level of sedation during recovery and at the hours after surgery.
Conclusion: Both ketamine and dexmedetomidine infusions during maintenance of anesthesia are effective in reducing acute postoperative pain effectively after PSF surgery.
Keywords: Acute postoperative pain, Dexmedetomidine hydrochloride, Ketamine hydrochloride, Posterior spinal fusion surgery
INTRODUCTION
One of the most challenging issues after surgery is providing appropriate pain control measures to enhance recovery of the patients.
Patients’ pain comes with unwanted side effects and complications. Meanwhile, the acute postoperative pain is considered as an annoying problem for patients after surgery and the severe pain can cause unfavorable hemodynamic and metabolic responses. About 21% of patients experience moderate-to-severe postoperative pain.[
Accordingly, the aim of this study was to compare the effects of ketamine versus dexmedetomidine infusion during surgery on the acute postoperative pain in PSF surgery.
MATERIALS AND METHODS
The study population consisted of patients candidate for PSF surgery who hospitalized in the hospital with which the authors are affiliated, in Tehran, Iran, during 2019– 2020. A double-blinded randomized clinical trial was conducted on 87 patients undergoing PSF surgery. The study protocol was approved by the Ethics Committee of Iran University of Medical Sciences (ethical code: IR.IUMS. FMD.REC.1397.213) and registered in Iranian Registry of Clinical Trials (IRCT) with ID: IRCT20180723040570N2. The sample size was estimated using the following formula. The sample size was 29 subjects per group. In the following relation, the values of Type I error (α) and Type II error (β) are estimated 5% and 2%, respectively. Furthermore, the values of standard deviations (SD) S1 and S2 are equal to 27.8 and 22, respectively, and the effect size (d) is 18.6 based on the values obtained for Fugl-Meyer Motor Scale in the study of Colette et al. (2011).[
After acquisition of informed consent and eligibility of patients according to inclusion and exclusion criteria, they were included in the study. The exclusion criteria included: PSF surgery due to traumatic fracture; those with a history of chronic mental disorder on medication; a history of systemic diseases such as heart failure and liver, renal, lung, and infectious diseases; history of hypertension (blood pressure higher than 140/90 mm Hg); and a history of diabetes (a fasting blood sugar level higher than 120 mg/dL) and history of addiction. Furthermore, patients with a history of seizure disorders and increased intracranial pressure, as well as patients with arrhythmia or heart rhythm disorders or AV block were excluded from the study.
Patients aged 18–70 years who were candidates for nontraumatic elective PSF surgery with a minimum of 2 and a maximum of 5 level involved rated AS: ASA1 or ASA2 were included in the study. The patients were randomly divided into three groups by block randomization: ketamine group (ROTEX-Germany) n = 29, dexmedetomidine group (MEDONX-Canada) n = 29, and control group n = 29. The patients took no sedatives or narcotics the night before surgery. In the operating room, standard monitoring was implemented (SAADAT ALBORZ B5, Iran) including electrocardiography, pulse oximetry, BIS monitoring (VISTA – Netherland), and noninvasive blood pressure monitoring and arterial line to measure their systolic and diastolic and mean arterial blood pressure. After IV cannulation with an 18 gauges needle, 0.5 cc/kg of lactated ringer was infused. Anesthesia protocol in all three groups was identical, including 25 μg/kg midazolam (EXIR, Iran), 3 μg/kg fentanyl (Caspian, Iran), 2 mg/kg propofol (B.BRAUN – Germany), 0.5 μg/kg atracurium (Aburaihan – Iran), and 1 mg/kg lidocaine (CASPIAN – Iran) for induction. All patients were given 0.1 mg/kg IV morphine (DAROUPAKSH – Iran) to reach the appropriate level of analgesia after changing their position and stabilizing of the vital signs. During the surgery, they received a standard dosage of fentanyl (1 μg/kg/h) so as to maintain the appropriate analgesia. Anesthesia maintenance regimen included 50–150 μg/kg propofol (B.BRAUN – Germany) with control of hemodynamic symptoms and the depth of anesthesia (by setting propofol dosage to maintain target BIS levels of 40–60) and 0.2 mg/kg atracurium/30 min (ABURAIHAN – Iran). During surgery, the first group received 0.2 mcg/kg/h dexmedetomidine infusion (MEDONEX – Canada) through infusion pump, the second group received 01 mg/kg/h ketamine infusion (R0TEX – Germany), and control group received the normal saline infusion used as a placebo. All patients had an arterial line and their blood pressure and heart rate were measured before and during surgery. More than 30% decrease in heart rate (compared to baseline resting heart rate) or heart rate of fewer than 50 beats/min was considered as bradycardia and more than 30% reduction in arterial pressure (compared to baseline mean arterial pressure [MAP]) MAP < 60 mmHg was regarded as hypotension. The protocol for bradycardia treatment was IV atropine 0.01 mg/kg body weight (CASPIAN – Iran) and those with hypotension, intermittent intravenous dose of 40 mcg phenylephrine (BB PHARMA – Iran) until the MAP reached above 60 mmHg. More than 30% increase in heart rate compared to baseline before surgery (with a target BIS range of 40–60 and IV fluid therapy based on standard protocol of 4-2-1) was considered as tachycardia and more than 30% increase in mean arterial pressure compared to baseline before surgery (with a target BIS range of 40–60 and IV fluid therapy based on standard calculations) was regarded as hypertension. For hemodynamic control in these cases, labetalol 2 mg/min and TNG 5 μg/min as a baseline were infused and increased to target levels. The total dose of propofol infused (Caspian, Iran) and the time of surgery was recorded. At the end of surgery, for pain control, all patients had (Accufuser CTx, South Korea), an autofuser/containig2g Apotel (EXIR – Iran) and 500 μg fentanyl (Caspian, Iran). Filled with up to 100 cc volume with hypertonic saline, the pump was set at an infusion rate of 4 cc/h for all patients. If the value of a visual analog pain scale (VAS) was >3 while receiving infusion, 0.3 mg/kg pethidine (CASPIAN – Iran) was injected. The amount of the opioids and number of injection prescribed for the patients were recorded during the first 24 h after surgery. Furthermore, the level of sedation (assessed by Ramsey scale) and pain intensity (assessed by VAS scale) were measured for patients during the recovery and at 2, 4, 6, 12, and 24 h after surgery, and their bradycardia and hypotension rates from induction to extubation were recorded and compared during surgery. Surgical team was identical in groups. For statistical analysis of the data, quantitative variables are expressed as means ± SD and qualitative variables as percentages. If the data were normally distributed, analysis of variance, or ANOVA, was used to compare the quantitative variables and if data were not normally distributed, the Kruskal-Wallis test was employed. The Chi-square test or Fisher’s exact test was used to compare the qualitative variables. Statistical analysis was performed using SPSS version 25. P < 0.05 was regarded as statistically significant.
RESULTS
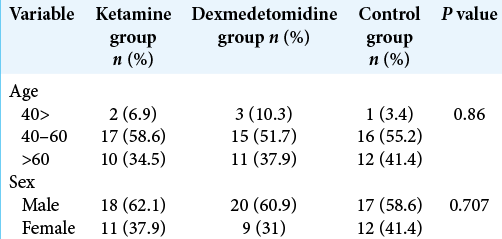
A total of 87 patients undergoing PSF surgery who hospitalized in Rasoul Akram Hospital in Tehran, Iran, during 2019–2020, were included in the study.
As shown in [
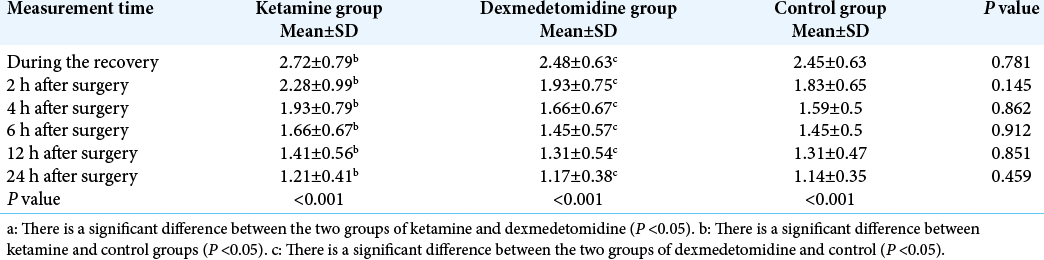
Based on the results of ANOVA and Kruskal-Wallis test, there was no significant difference among the groups in terms of level of sedation during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P > 0.05).The repeated measures ANOVA results showed that intragroup measurement of level of sedation was significantly decreased in the three groups during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P < 0.05) [
There was no significant difference among the groups in terms of level of sedation during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P > 0.05).
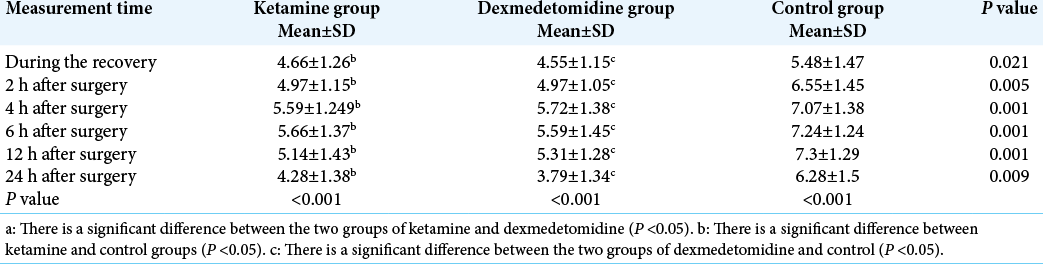
The results of ANOVA and Kruskal-Wallis test showed that there was a significant difference among the groups with respect to the pain intensity during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P < 0.05). Furthermore, a significant difference was observed between the ketamine and control groups with respect to the pain intensity during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P < 0.05). Moreover, there was a significant difference between dexmedetomidine and control groups in terms of the pain intensity during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P < 0.05). The repeated measures ANOVA results indicated that there was a significant difference among the intragroups with respect to the pain intensity during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P < 0.05) [
As shown in [
As shown in [
DISCUSSION
The results of the present study demonstrate that there was a significant difference among the groups with respect to the pain intensity during the recovery and at 2, 4, 6, 12, and 24 h after surgery (P < 0.05). However, there were no significant differences between ketamine and dexmedetomidine groups with respect to the pain intensity. Furthermore, there was a significant difference among the groups with respect to the amount and number of opioids prescribed during the recovery and at 2, 4, 6, 12, and 24 h after surgery. Moreover, there was no significant difference among the groups in terms of level of sedation during the recovery and at 2, 4, 6, 12, and 24 h after surgery.[
The result of the study of Rahimzadeh et al. showed that dexmedetomidine had a significant lowering impact on intraoperative blood pressure and heart rate compared to remifentanil (P < 0.001). The mean of postextubation and recovery pain score in patients taking remifentanil was significantly higher than patients taking dexmedetomidine (P < 0.05).[
CONCLUSION
Both ketamine and dexmedetomidine are effective in reducing the acute pain after PSF surgery. The lack of significant difference between the ketamine and dexmedetomidine may be due to a low dose of the dexmedetomidine. Therefore, it is recommended to examine the different doses of dexmedetomidine or combination of ketamine and dexmedetomidine in the future studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors wish to thank the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC), Iran University of Medical Science for its editorial assist.
References
1. Abd Aziz N, Chue MC, Yong CY, Hassan Y, Awaisu A, Hassan J. Efficacy and safety of dexmedetomidine versus morphine in post-operative cardiac surgery patients. Int J Clin Pharm. 2011. 33: 150-4
2. Anger KE, Szumita PM, Baroletti SA, Labreche MJ, Fanikos J. Evaluation of dexmedetomidine versus propofol-based sedation therapy in mechanically ventilated cardiac surgery patients at a tertiary academic medical center. Crit Pathw Cardiol. 2010. 9: 221-6
3. Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): A randomised placebo-controlled trial. Lancet Neurol. 2011. 10: 123-30
4. Gharaei B, Jafari A, Aghamohammadi H, Kamranmanesh M, Poorzamani M, Elyassi H. Opioid-sparing effect of preemptive bolus low-dose ketamine for moderate sedation in opioid abusers undergoing extracorporeal shock wave lithotripsy: A randomized clinical trial. Anesth Analg. 2013. 116: 75-80
5. Gramke HF, de Rijke JM, van Kleef M, Raps F, Kessels AG, Peters ML. The prevalence of postoperative pain in a cross-sectional group of patients after day-case surgery in a university hospital. Clin J Pain. 2007. 23: 543-8
6. Ketonis C, Ilyas AM, Liss F. Pain management strategies in hand surgery. Orthop Clin North Am. 2015. 46: 399-408
7. Martin E, Narjoz C, Decleves X, Labat L, Lambert C, Loriot MA. Dextromethorphan analgesia in a human experimental model of hyperalgesia. Anesthesiology. 2019. 131: 356-68
8. Mitra R, Prabhakar H, Rath GP, Bithal PK, Khandelwal A. Comparison of small dose ketamine and dexmedetomidine infusion for postoperative analgesia in spine surgery--a prospective randomized double-blind placebo controlled study. J Neurosurg Anesthesiol. 2016. 28: 27-31
9. Parikh B, Maliwad J, Shah VR. Preventive analgesia: Effect of small dose of ketamine on morphine requirement after renal surgery. J Anaesthesiol Clin Pharmacol. 2011. 27: 485-8
10. Rahimzadeh P, Faiz SH, Alimian M, Erdi AM. Remifentanil versus dexmedtomidine for posterior spinal fusion surgery. Med J Islam Repub Iran. 2015. 29: 215
11. Reichert MG, Jones WA, Royster RL, Slaughter TF, Kon ND, Kincaid EH. Effect of a dexmedetomidine substitution during a nationwide propofol shortage in patients undergoing coronary artery bypass graft surgery. Pharmacotherapy. 2011. 31: 673-7
12. Samin J, Collange O, Pourtalès MC, Ravaz T, Calon B, Pottecher T. Assessment of quality in day-case hand surgery. Ann Fr Anesth Reanim. 2009. 28: 735-42
13. Shang AB, Gan TJ. Optimising postoperative pain management in the ambulatory patient. Drugs. 2003. 63: 855-67
14. Suzuki M. Role of N-methyl-D-aspartate receptor antagonists in postoperative pain management. Curr Opin Anaesthesiol. 2009. 22: 618-22
15. Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J. Dexmedetomidine infusion during laparoscopic bariatric surgery: The effect on recovery outcome variables. Anesth Analg. 2008. 106: 1741-8
16. Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010. 83: 11-25
17. Wu L, Huang X, Sun L. The efficacy of N-methyl-D-aspartate receptor antagonists on improving the postoperative pain intensity and satisfaction after remifentanil-based anesthesia in adults: A meta-analysis. J Clin Anesth. 2015. 27: 311-24