- Department of Neurosurgery, Yokohama Rosai Hospital, Yokohama, Japan.
Correspondence Address:
Misaki Kamogawa, Department of Neurosurgery, Yokohama Rosai Hospital, Yokohama, Japan.
DOI:10.25259/SNI_802_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Misaki Kamogawa, Takashi Shuto, Shigeo Matsunaga. Effects of two different radiotherapies for craniopharyngiomas using stereotactic radiosurgery/ stereotactic radiotherapy or fractionated stereotactic radiotherapy. 02-Dec-2022;13:563
How to cite this URL: Misaki Kamogawa, Takashi Shuto, Shigeo Matsunaga. Effects of two different radiotherapies for craniopharyngiomas using stereotactic radiosurgery/ stereotactic radiotherapy or fractionated stereotactic radiotherapy. 02-Dec-2022;13:563. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12037
Abstract
Background: Numerous studies have reported about good tumor control with both stereotactic radiosurgery (SRS) and fractionated stereotactic radiotherapy (FSRT) for residual and recurrent craniopharyngiomas, but no studies have reported on the appropriate use of different types of radiation modalities. This study aimed to report the outcomes of SRS/stereotactic radiotherapy (SRT) or FSRT and compare tumor control in a single center.
Methods: From 2014 when TrueBeamTM STx with Novalis was introduced in our hospital to 2021, 21 patients underwent SRS/SRT or FSRT with gamma knife surgery (GKS) and Novalis. We have selected the radiation modalities considering mainly the distance of the optic nerve and chiasm. Imaging and clinical follow-up data were sent and reviewed.
Results: The mean age was 52 years and there were 11 men. Of the 21 total patients, three experienced SRS (GKS, 50% isodose 12–15 Gy), five underwent SRT (GKS or Novalis, 19.5–24 Gy 3 fractions), and 13 patients underwent FSRT (Novalis, 54 Gy 30 fractions). The median follow-up was 32.6 (range 17–44) months after SRS/SRT and 34.0 (range 4–61) months after FSRT. In the SRS/SRT group, the mean tumor volume decreased from 1.103 to 0.131 cm3 (P 3 (P
Conclusion: Craniopharyngioma can be expected to have very good tumor control by selecting SRS/SRT or FSRT depending on the distance between the optic nerve and the tumor.
Keywords: Craniopharyngioma, Fractionated stereotactic radiotherapy, Gamma knife surgery, Long-term tumor control, Optic nerve
INTRODUCTION
Craniopharyngiomas are epithelial tumors that arise from squamous epithelial remnants of the Rathke pouch. Despite their histologically benign and growing slowly, they often cause clinical disorders such as visual and hypothalamic impairment and hypopituitarism. Its gold standard of treatment is surgical resection;[
Both stereotactic radiosurgery (SRS).[
From 2014 when Novalis was introduced in our hospital, we have selected SRS/stereotactic radiotherapy (SRT) with GKS or FSRT with Novalis considering mainly the distance of the optic nerve and chiasm. To the best of our knowledge, this is the first comparative single-center study of the treatment of craniopharyngiomas using GKS and linac-based stereotactic irradiation. We present the effect of this different RT and present an appropriate patient-specific treatment plan.
MATERIALS AND METHODS
From April 2014 to December 2021, 21 patients with histologically confirmed craniopharyngioma were treated for residual or recurrent craniopharyngioma using GKS or linac at Yokohama Rosai Hospital. In selecting the optimal dose and number of fractions, we have placed the highest priority on the distance between the optic nerve/optic chiasm and the tumor. The maximum dose applied to the optic nerve and chiasm was ≤8 gray (Gy). Patient’s treatment was selected according to the following policies:
SRS/SRT was selected when the distance was far from several millimeters FSRT was selected when the tumor is touching or compressing the optic tracts Other selection criteria are as follows: Multisession GKS was performed when the residual or recurrence craniopharyngioma, especially the cyst component, had been growing rapidly.
Both SRS and SRT are strategies based on the concept of increasing the single dose or dose per fraction and shortening the total treatment period. FSRT, on the other hand, is a strategy based on the concept of reducing the dose per fraction, giving priority to tissue tolerance, and achieving tumor control by performing multiple irradiations. For this reason, we combined SRS and SRT into one strategy.
GKS technique
GKS was performed using a Leksell Gamma Knife Perfexion (Elekta Instrument AB, Stockholm, Sweden) and multisession GKS was performed with the Gamma Knife Extend System.[
FSRT technique
The gross tumor volume was defined as the contrast-enhanced lesion on T1-weighted MRI. The clinical target volume (CTV) was expanded to a larger planning target volume (PTV), which included the CTV with a safety margin of 1–2 mm (median 1). HybridArc plans[
Imaging and clinical follow-up information were sent and reviewed at our center. Standard informed consent relating to summarizing radiation effects on craniopharyngiomas was obtained for each patient. We focused on imaging data, visual acuity, visual field, and endocrine function before and after RT. Computed tomography (CT) is performed if MRI is not available. Follow-up examinations were scheduled every 3 months to 1 year and obtained directly or from referring physicians. The tumor volume was calculated from pre- and post-treatment MRI or CT scans by contouring the lesion on each slice of a contrast-enhanced, T1-weighted MRI axial scan using the “volume” function in the “measurements” window of the GammaPlan software. Each tumor was classified into five groups after radiation:[
Statistical analysis
Statistical analyses were performed using R programming.[
RESULTS
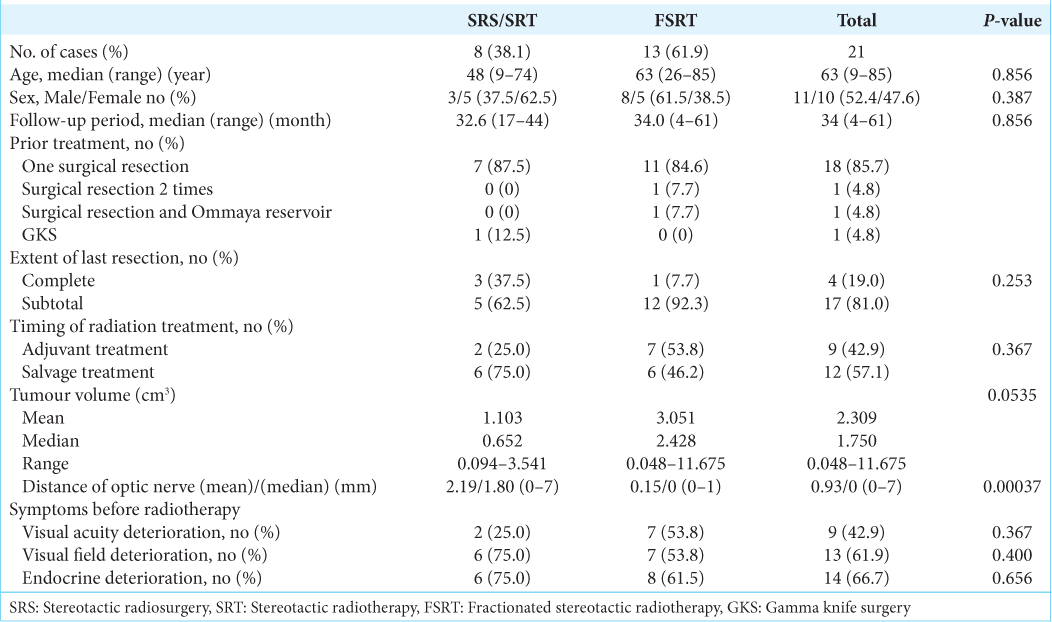
Patient characteristics are summarized in
Three patients experienced SRS (GKS, 50% isodose 12–15 Gy), five underwent SRT (GKS or linac, 19.5–24 Gy for three fractions), and 13 underwent FSRT (linac, 54Gy 30 fractions). One pediatric patient received SRS under general anesthesia. The median follow-up was 32.6 (range 17–44) months after SRS/SRT and 34.0 (range 4–61) months after FSRT.
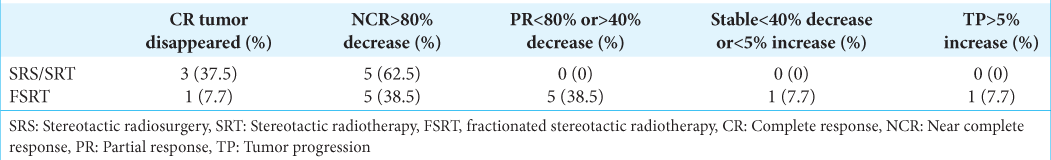
In the SRS/SRT group, the final radiation outcomes were CR in 37.5% and NCR in 62.5%. In the FSRT group, the outcomes were CR in 7.7%, NCR in 38.5 %, PR in 38.5%, stable in 7.7%, and TP in 7.7% of the cases [
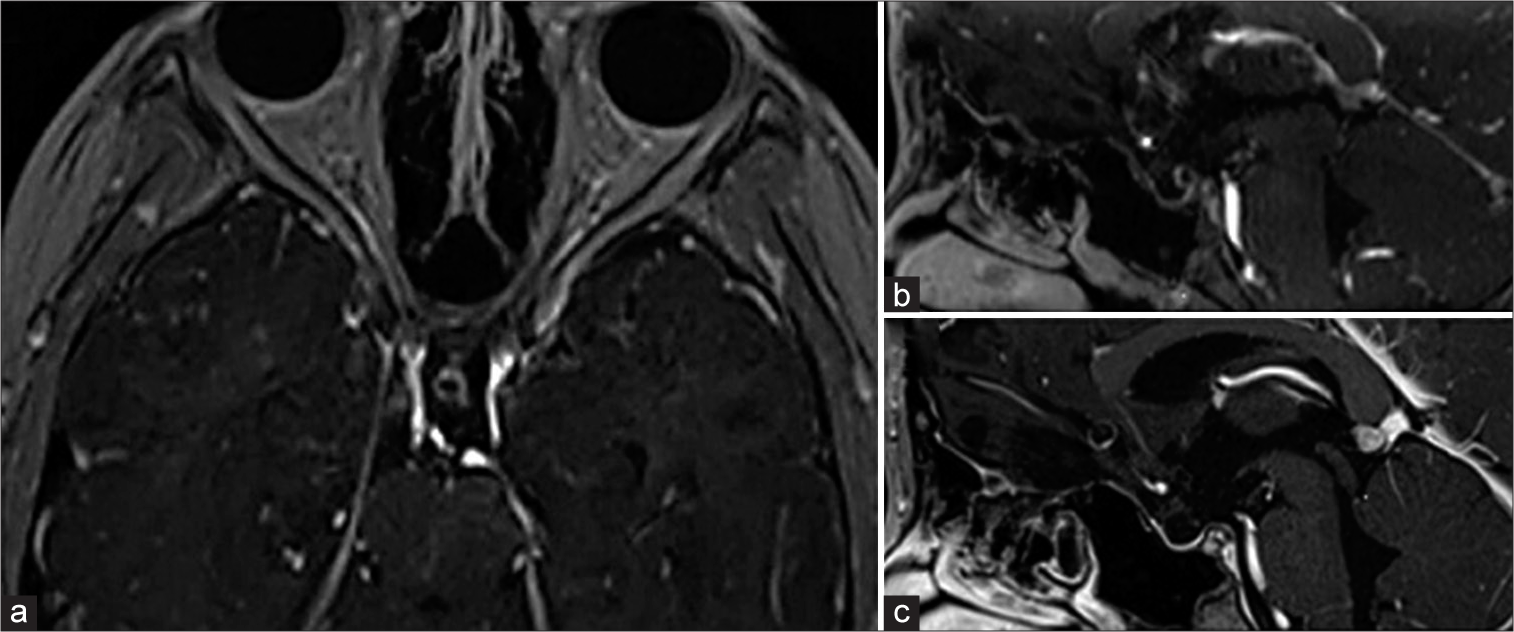
Figure 2:
A 10-years-old girl underwent craniotomy 33 months ago. The recurrent tumor was on the dorsal side of the optic chiasm and the distance between the tumour and chiasma was 2 mm (a and b). We selected stereotactic radiosurgery under general anesthesia. No visual impairment occurred within the 17-month follow-up period, the tumor disappeared (c).
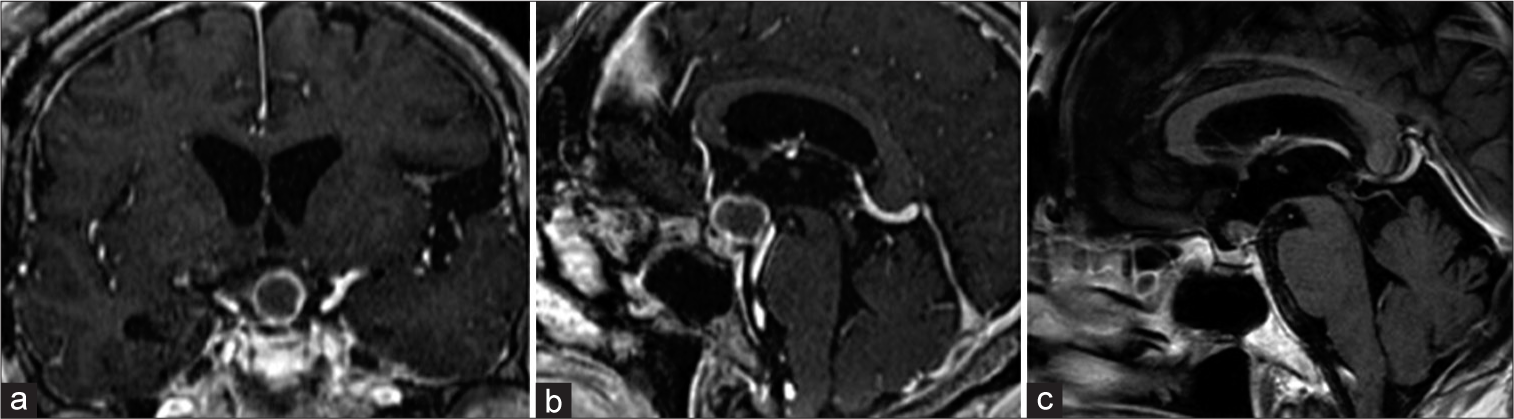
Figure 3:
A 65-year-old woman received radiation therapy 32 months after surgery. The residual tumor was cystic but did not grow fast. The tumor compressed the optic nerve and it was relatively large (a and b), so we selected 30 fractions, and tumor control was great 54 months after fractionated stereotactic radiotherapy (c).
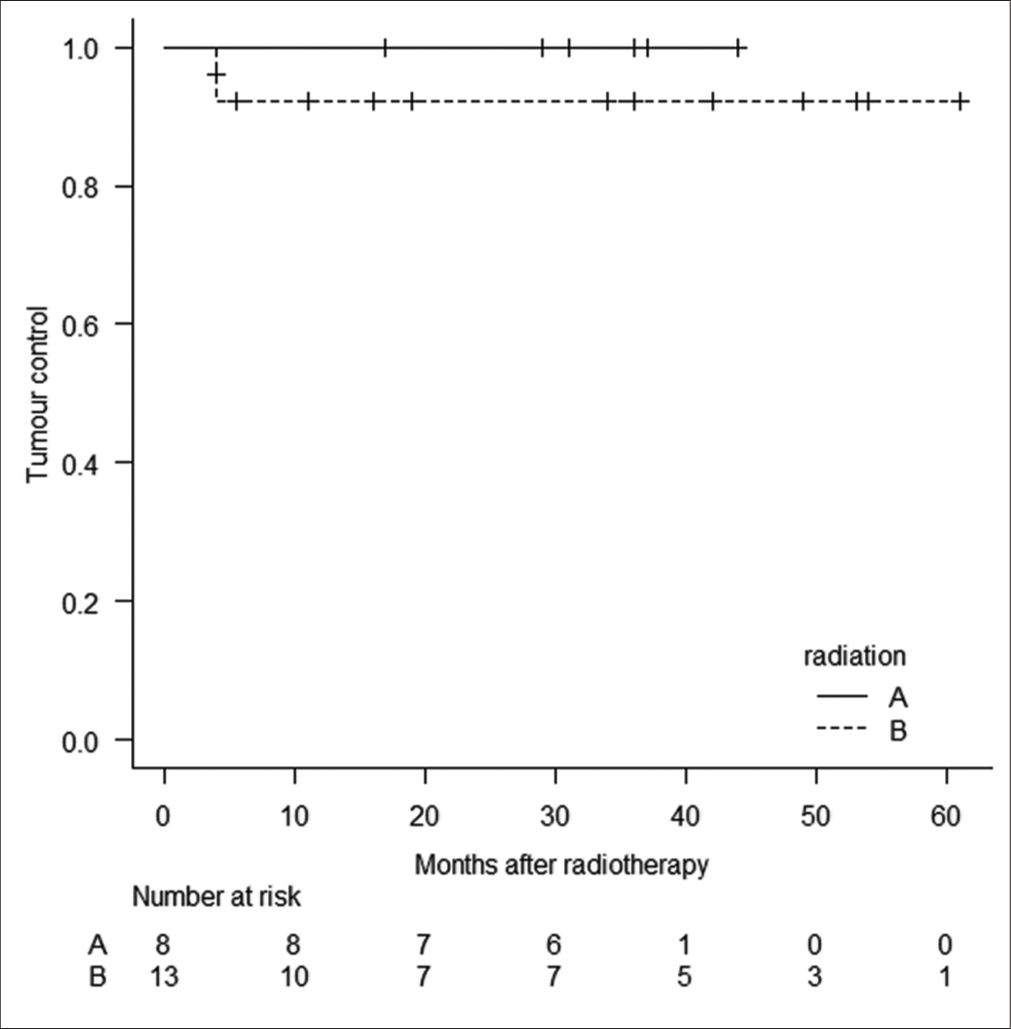
Both types of RT result in great tumor control [
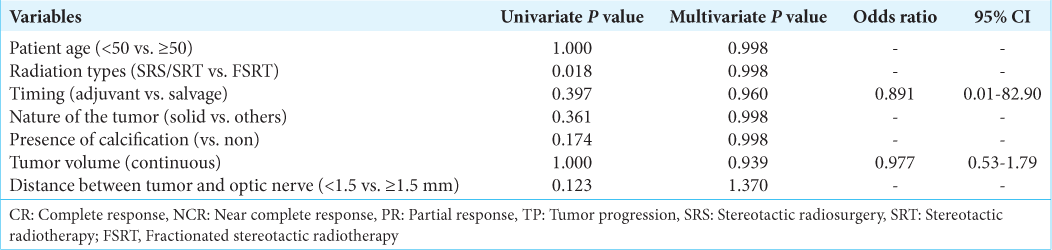
No radiation-induced optic neuropathy, acute toxicity, and other adverse events such as radiation necrosis and cyst formation occurred after SRS/SRT and FSRT. During follow-up, visual acuity was improved in 1 (4.8%) patient who underwent FSRT, and visual field defects were improved in 3 (14.3%) patients. Pituitary function remained unchanged during follow-up. Because formal neurocognitive testing was not obtained from all patients, data concerning cognitive toxicity could not be retrospectively extracted. During follow-up, further treatment included GKS was not needed. Only one patient with enlarged tumor died from another factor and follow-up period was 4 months.
DISCUSSION
Craniopharyngiomas are histologically benign and are slowly progressive, but GTR is challenging because of the anatomical position, that is, the tumor is located very close to the optic apparatus, pituitary, and critical vascular structures. RT such as GKS and linac has been proven to be effective as salvage methods for residual and recurrent craniopharyngiomas. This tumor has been considered good candidates for RT; however, there are several clinical challenges to achieve a long-term tumor control.
First, the sufficient dose to achieve good long-term tumor control without causing visual dysfunction is not yet clarified. GKS has been reported to be an effective management option and studies have suggested 5-year progression-free survival rate of 72.1–90.3%.[
On the contrary, using FSRT rather than SRS, it becomes possible to treat tumors safety when they are in contact with the optic nerve or when it is difficult to identify the optic pathway on the image. FSRT also minimizes toxicity to normal tissues and enables treatment of large tumors. A reduction of marginal dose can be possible compared with before, which brought a smaller PTV and higher doses to the target with normal tissues were spared.[
As mentioned above, FSRT is much effective in the case that it is difficult to identify the optic pathway. However, tumors with cystic growth may be challenging to control because FSRT must take a long time. The previous studies have indicated tumor enlargement during or after the treatment, especially in craniopharyngiomas with cystic components.[
Patterns of cystic changes are classified into two types: early yet transient growth and slowly progressive and late growth. Early cyst growth seems to be appeared within 3 months during or after RT, and frequent surveillance imaging during RT is needed. This type is temporary and spontaneous decompression is obtained over time, whereas late cyst growth was related to visual and hypothalamic toxicities (P = 0.009 and 0.04) on the multivariate analysis.[
A smaller cyst volume should be associated with better tumor control.[
In this study, only one tumor was identified to have enlarged and categorized as TP, which was mainly a cystic lesion. We selected FSRT because the tumor compressed the optic nerve. In the case that the cystic component enlarged, the CTV margin was determined as 2 mm. Moreover, the patient had Ommaya reservoir and the cystic component was punctured during the treatment to reduce the volume. Four months later, MRI showed tumor progression compared with before RT. Although the observation period was short, it was the latest evaluation, and we judged it to be uncontrolled.
In this study, we have selected types of radiation based on the distance between the tumor and the optic nerve. Improved RT techniques over time have brought remarkable results in tumor control and enable treatment more safety. We can achieve long-term tumor control while maintaining the quality of life in many cases by changing the treatment and intervention according to each case. To the best of our knowledge, this is the first report to describe treatment options for gamma knife and linac in a single center.
Limitation
This study has several limitations mainly due to its small number of patients, especially pediatrics. We have not yet encountered cases requiring general anesthesia for FSRT in children. No patients developed a new pituitary deficiency because few pediatric cases were reported in this report. Moreover, visual disturbance generally occurs within 3 years after radiation,[
CONCLUSION
Craniopharyngiomas are radiosensitive. The distance between the tumor and the optic nerve is the most important factor in treatment selection. The cystic component, speed of the tumor growth, and general condition of the patient may affect treatment choices. Appropriate management and selection of irradiation methods tailored to each patient appeared to provide a better long-term tumor control.
Consent to participate
For this type of study, written consent was not required. Standard informed consent was obtained and this study was reviewed and approved by the Institutional Review Board of Yokohama Rosai Hospital (# 2022-18).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We thank everyone who contributed to this project, especially those who provided us with the data.
References
1. Ajithkumar T, Mazhari AL, Stickan-Verfurth M, Kramer PH, Fuentes CS, Lambert J. Proton therapy for craniopharyngioma-an early report from a single European centre. Clin Oncol (R Coll Radiol). 2018. 30: 307-16
2. Astradsson A, Rosenschold PM, Feldt-Rasmussen U, Poulsgaard L, Wiencke AK, Ohlhues L. Visual outcome, endocrine function and tumor control after fractionated stereotactic radiation therapy of craniopharyngiomas in adults: Findings in a prospective cohort. Acta Oncol. 2017. 56: 415-21
3. Bishop AJ, Greenfield B, Mahajan A, Paulino AC, Okcu MF, Allen PK. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: Multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. 2014. 90: 354-61
4. Chung WY, Pan DH, Shiau CY, Guo WY, Wang LW. Gamma knife radiosurgery for craniopharyngiomas. J Neurosurg. 2000. 93: 47-56
5. Dho YS, Kim YH, Kim JW, Park CK, Chung HT, Kim SK. Optimal strategy of gamma knife radiosurgery for craniopharyngiomas. J Neurooncol. 2018. 140: 135-43
6. Harrabi SB, Adeberg S, Welzel T, Rieken S, Habermehl D, Debus J. Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: Maximal tumor control with minimal side effects. Radiat Oncol. 2014. 9: 203
7. Hasegawa T, Kobayashi T, Kida Y. Tolerance of the optic apparatus in single-fraction irradiation using stereotactic radiosurgery: Evaluation in 100 patients with craniopharyngioma. Neurosurgery. 2010. 66: 688-95
8. Hong CS, Omay SB. The role of surgical approaches in the multi-modal management of adult craniopharyngiomas. Curr Oncol. 2022. 29: 1408-21
9. Iwata H, Sato K, Nomura R, Tabei Y, Suzuki I, Yokota N. Long-term results of hypofractionated stereotactic radiotherapy with CyberKnife for growth hormone-secreting pituitary adenoma: evaluation by the Cortina consensus. J Neurooncol. 2016. 128: 267-75
10. Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013. 48: 452-8
11. Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, Ansorge O. Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf). 2005. 62: 397-409
12. Laville A, Coutte A, Capel C, Maroote J, Lefranc M. Dosimetric and volumetric outcomes of combining cyst puncture through an Ommaya reservoir with index-optimized hypofractionated stereotactic radiotherapy in the treatment of craniopharyngioma. Clin Transl Radiat Oncol. 2020. 23: 66-71
13. Lee MH, Kim SH, Seoul HJ, Nam DH, Lee JI, Park K. Impact of maximal safe resection on the clinical outcome of adults with craniopharyngiomas. J Clin Neurosci. 2012. 19: 1005-8
14. Li J, To D, Gunn V, Shi W, Yu Y, Liu H. Evaluation of hybrid arc and volumetric-modulated arc therapy treatment plans for fractionated stereotactic intracranial radiotherapy. Technol Cancer Res Treat. 2018. 17: 1-6
15. Liu X, Yu Q, Zhang Z, Zhang Y, Li Y, Liu D. Same-day stereotactic aspiration and Gamma Knife surgery for cystic intracranial tumors. J Neurosurg. 2012. 117: 45-8
16. Losa M, Pieri V, Bailo M, Gagliardi F, Barzaghi LR, Gioia L. Single fraction and multisession Gamma Knife radiosurgery for craniopharyngioma. Pituitary. 2018. 21: 499-506
17. Merchant TE, Kun LE, Hua CH, Wu S, Xiong X, Sanford RA. Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 2013. 85: e187-92
18. Milano MT, Grimm J, Soltys SG, Yorke E, Moiseenko V, Tome WA. Single-and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol Biol Phys. 2021. 110: 87-99
19. Mohamed Ali A, Mathis T, Bensadoun RJ, Thariat J. Radiation induced optic neuropathy: Does treatment modality influence the risk?. Bull Cancer. 2019. 106: 1160-6
20. Ogino A, Niranjan A, Kano H, Flickinger JC, Lunsford LD. Optimizing stereotactic radiosurgery in patients with recurrent or residual craniopharyngiomas. J Neurooncol. 2021. 154: 113-20
21. Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N. Pediatric craniopharyngiomas classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007. 106: 3-12
22. Schlesinger D, Xu Z, Taylor F, Yen CP, Sheehan J. Interfraction and intrafraction performance of the Gamma Knife Extend system for patient positioning and immobilization. J Neurosurg. 2012. 117: 217-24
23. Schulz-Ertner D, Frank C, Herfarth KK, Rhein B, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy for craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2002. 54: 1114-20
24. Shi Z, Esiashvili N, Janss AJ, Mazewski CM, MacDonald TJ, Wrubel DM. Transient enlargement of craniopharyngioma after radiation therapy: Pattern of magnetic resonance imaging response following radiation. J Neurooncol. 2012. 109: 349-55
25. Tishler RB, Loeffler JS, Lunsford LD, Duma CM, Alexander E, Kooy HM. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Pyhs. 1993. 27: 215-21
26. Tsugawa T, Kobayashi T, Hasegawa T, Iwai Y, Matsunaga S, Yamamoto M. Gamma knife surgery for residual or recurrent craniopharyngioma after surgical resection: A multi-institutional retrospective study in Japan. Cureus. 2020. 12: e6973
27. Van Effenterre R, Boch AL. Craniopharyngioma in adults and chirldren: A study of 122 surgical cases. J Neurosurg. 2002. 97: 3-11