- Department of Neurosurgery, Iwaki City Medical Center, Iwaki, Japan.
- Department of Radiology, Iwaki City Medical Center, Iwaki, Japan.
- Department of Neurosurgery, Khonan Hospital, Sendai, Japan.
- Department of Neurosurgery, Tohoku University Graduate School of Medicine, Sendai, Japan.
Correspondence Address:
Ryuzaburo Kochi, Department of Neurosurgery, Iwaki City Medical Center, Iwaki, Japan.
DOI:10.25259/SNI_200_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryuzaburo Kochi1, Yasuhiro Suzuki1, Hiroshi Yamazaki2, Takashi Aikawa1, Hidenori Endo3, Teiji Tominaga4. Efficacy of repeat arterial spin labeling for angiogram-negative ruptured micro-arteriovenous malformation: A case report. 31-Mar-2023;14:119
How to cite this URL: Ryuzaburo Kochi1, Yasuhiro Suzuki1, Hiroshi Yamazaki2, Takashi Aikawa1, Hidenori Endo3, Teiji Tominaga4. Efficacy of repeat arterial spin labeling for angiogram-negative ruptured micro-arteriovenous malformation: A case report. 31-Mar-2023;14:119. Available from: https://surgicalneurologyint.com/surgicalint-articles/12223/
Abstract
Background: Diagnosing ruptured micro-arteriovenous malformation (AVM) could be difficult using digital subtraction angiography (DSA) in the acute stage, and a repeat DSA is recommended in DSA-negative cases. Arterial spin labeling (ASL) is a useful noninvasive tool for detecting AVM, but the efficacy of a repeat ASL for DSA and ASL-negative ruptured micro-AVM in the acute stage is unclear. Here, we report a case of ruptured micro-AVM that was not detected in the acute stage by ASL but in the chronic stage by ASL.
Case Description: A 43-year-old man developed right upper-extremity paralysis, and computed tomography (CT) revealed a left frontal lobe hemorrhage. Magnetic resonance imaging, including ASL, CT angiography, and DSA, showed no abnormal findings associated with hemorrhage in the acute stage. The second ASL 93 days after the hemorrhage showed a high signal on the cortical vein of the left frontal lobe and superior sagittal sinus, and subsequent DSA detected a micro-AVM in the left precentral gyrus.
Conclusion: Repeat ASL is less invasive and useful for detecting micro-AVMs which showed no findings on ASL and DSA in the acute stage.
Keywords: Arterial blood, Arterial spin labeling, Digital subtraction angiography, Hematoma, Micro-arteriovenous malformation
INTRODUCTION
Digital subtraction angiography (DSA) is the gold standard for the definitive diagnosis of arteriovenous malformations (AVM). However, some cases of ruptured AVM with hematoma do not show any findings on DSA in the acute stage.[
CASE REPORT
A 43-year-old male with no history of hypertension, hyperlipidemia, or diabetes mellitus was admitted to our hospital with sudden onset of the right upper-extremity paralysis. On admission, computed tomography (CT) revealed left frontal lobe hemorrhage [
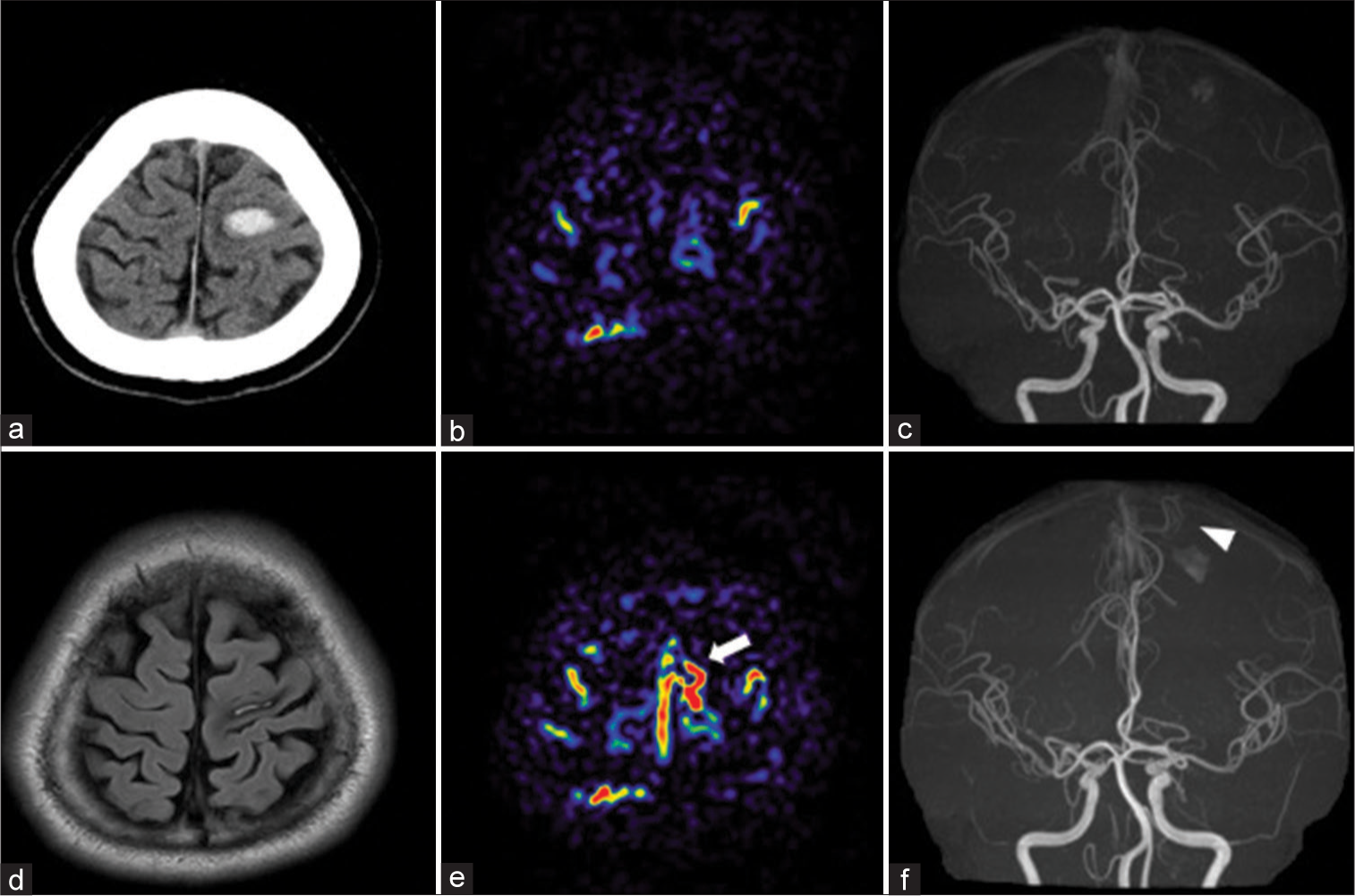
Figure 1:
(a) Computed tomography (CT) on admission. Magnetic resonance imaging (b and c) 3 days and (d-f) 93 days after onset (a) CT: Hematoma in the left primary motor cortex. (b) Arterial spin labeling (ASL): No obvious high signal around the hematoma. (c) Magnetic resonance angiography (MRA): No abnormal vessel around the hematoma (d) Flow-attenuated inverted recovery: Shrinkage of hematoma. (e) ASL: High focal signal on the convexity of the left frontal lobe and suprasagittal sinus (white arrow), which was not seen in previous ASL. (f) MRA showing a newly depicted vessel (white arrowhead) flowing in the direction of the hematoma.
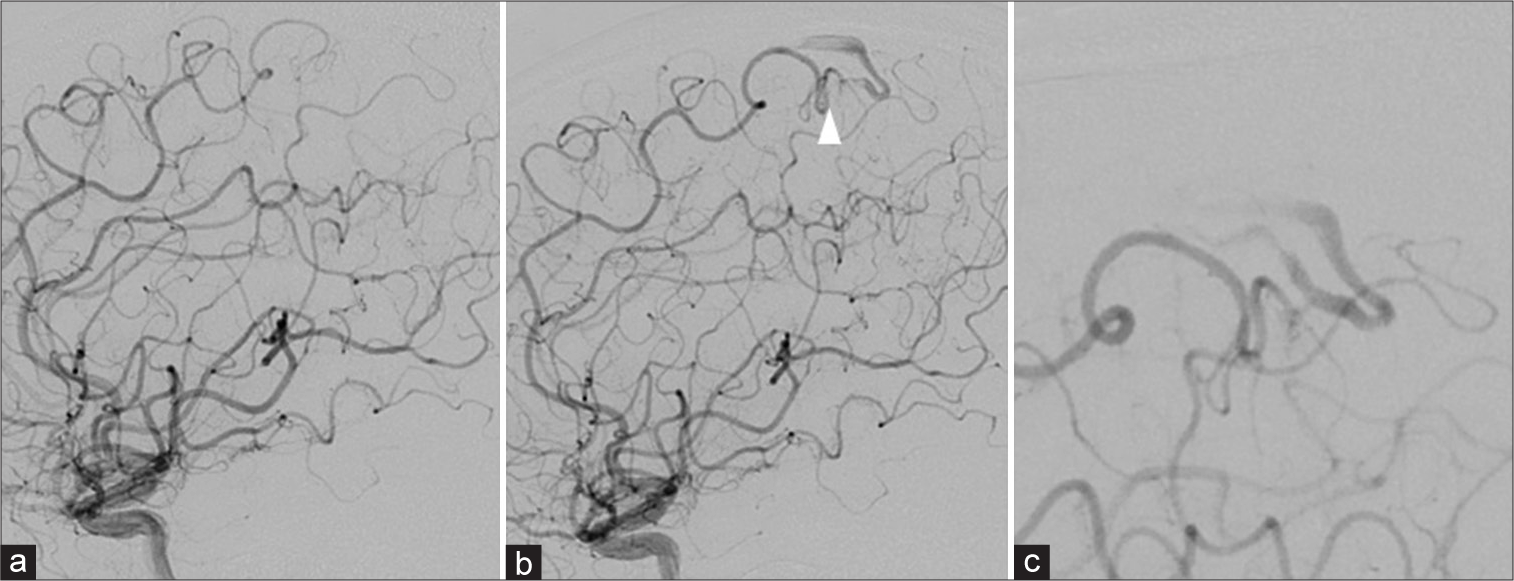
Figure 2:
Digital subtraction angiography (DSA). (a) 1 day after onset (b) 93 days after onset (a) abnormal vessel and early venous filling were not observed. (b) Micro-arteriovenous malformation (AVM) (white arrowhead) was observed, which was not seen on DSA 5 days after onset. (c) Enlarged micro-AVM image. The micro-AVM comprised a 6 mm nidus with a single feeding of a branch of the left anterior parietal artery. A draining vein arose from the posterior side of the nidus and flowed toward the superior sagittal sinus through the cortex vein on the frontal lobe.
DISCUSSION
In ASL, arterial blood is magnetically labeled at the cervical segment, and brain images are acquired following a short delay to allow the labeled arterial blood to flow through the cerebral arteries and into the capillary bed.[
According to the previous reports, acute-stage DSA may not reveal a vascular malformation in some cases of ruptured AVM with hematoma.[
CONCLUSION
Ruptured micro-AVMs might be misdiagnosed during acute-stage examinations. Repeat ASL is less invasive and useful for detecting micro-AVMs which showed no findings on ASL and DSA in the acute stage.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alen JF, Lagares A, Paredes I, Campollo J, Navia P, Ramos A. Cerebral microarteriovenous malformations: A series of 28 cases. J Neurosurg. 2013. 119: 594-602
2. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 1: Technique and artifacts. AJNR Am J Neuroradiol. 2008. 29: 1228-34
3. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 2: Hypoperfusion patterns. AJNR Am J Neuroradiol. 2008. 29: 1235-41
4. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, Part 3: Hyperperfusion patterns. AJNR Am J Neuroradiol. 2008. 29: 1428-35
5. Detre JA, Alsop DC, Vives LR, Maccotta L, Teener JW, Raps EC. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology. 1998. 50: 633-41
6. Hino A, Fujimoto M, Yamaki T, Iwamoto Y, Katsumori T. Value of repeat angiography in patients with spontaneous subcortical hemorrhage. Stroke. 1998. 29: 2517-21
7. Hodel J, Leclerc X, Kalsoum E, Zuber M, Tamazyan R, Benadjaoud MA. Intracranial arteriovenous shunting: Detection with arterial spin-labeling and susceptibility-weighted imaging combined. AJNR Am J Neuroradiol. 2017. 38: 71-6
8. Kochi R, Endo H, Uchida H, Kawaguchi T, Omodaka S, Matsumoto Y. Efficacy of arterial spin labeling for detection of the ruptured micro-arteriovenous malformation: Illustrative cases. J Neurosurg Case Lessons. 2022. 3: CASE21597
9. Le TT, Fischbein NJ, Andre JB, Wijman C, Rosenberg J, Zaharchuk G. Identification of venous signal on arterial spin labeling improves diagnosis of dural arteriovenous fistulas and small arteriovenous malformations. AJNR Am J Neuroradiol. 2012. 33: 61-8
10. Ogilvy CS, Heros RC, Ojemann RG, New PF. Angiographically occult arteriovenous malformations. J Neurosurg. 1988. 69: 350-5
11. Shimogawa T, Morioka T, Sayama T, Haga S, Kanazawa Y, Murao K. The initial use of arterial spin labeling perfusion and diffusion-weighted magnetic resonance images in the diagnosis of nonconvulsive partial status epileptics. Epilepsy Res. 2017. 129: 162-73
12. Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: Clinical applications in the brain. J Magn Reson Imaging. 2015. 41: 1165-80
13. Wolf RL, Wang J, Detre JA, Zager EL, Hurst RW. Arteriovenous shunt visualization in arteriovenous malformations with arterial spin-labeling MR imaging. AJNR Am J Neuroradiol. 2008. 29: 681-7
14. Zaharchuk G. Arterial spin-labeled perfusion imaging in acute ischemic stroke. Stroke. 2014. 45: 1202-7