- Department of Neurosurgery, Tanta University, Tanta, Gharbia, Egypt.
DOI:10.25259/SNI_278_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Abdelaziz Elsharkawy, Essam Ahmed Abdelhameed. Efficacy of translamina terminalis ventriculostomy tube in prevention of chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. 12-Sep-2020;11:283
How to cite this URL: Ahmed Abdelaziz Elsharkawy, Essam Ahmed Abdelhameed. Efficacy of translamina terminalis ventriculostomy tube in prevention of chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. 12-Sep-2020;11:283. Available from: https://surgicalneurologyint.com/surgicalint-articles/10257/
Abstract
Background: Chronic shunt-dependent hydrocephalus is still a common complication after aneurysmal SAH (aSAH) and is associated with increased morbidity. Pathology of chronic shunt-dependent hydrocephalus after aSAH is complex and multifactorial which makes its prevention challenging. We thought to evaluate whether external ventricular drainage (EVD) through fenestrated lamina terminalis would decrease the rate of chronic shunt-dependent hydrocephalus after aSAH.
Methods: A retrospective analysis of 68 consecutive patients with aSAH who underwent microsurgical clipping of the ruptured aneurysm. Patients were divided into two groups: Group A included patients with lamina terminalis fenestration without insertion of ventriculostomy tube and Group B included patients with EVD through fenestrated lamina terminalis. Demographic, clinical, radiological, and outcome variables were compared between groups.
Results: Group A comprised 29 patients with mean age of 47.8 years and Group B comprised 39 patients with mean age of 46.6 years. Group B patients had statistically significant (P
Conclusion: EVD through fenestrated lamina terminalis is safe and may be effective in decreasing the incidence of chronic shunt-dependent hydrocephalus after aSAH.
Keywords: Aneurysmal subarachnoid hemorrhage, External ventricular drainage, Lamina terminalis, Shunt- dependent hydrocephalus
INTRODUCTION
Chronic shunt-dependent hydrocephalus is a common consequence after aneurysmal subarachnoid hemorrhage (aSAH) with reported incidence of 8.9–48%. It contributes largely to patient long-term morbidity, hospital readmission, and health-care economic burden occurring after aSAH. Several factors have been identified to predict the development of chronic hydrocephalus after aSAH including radiological findings as hydrocephalus at presentation, intraventricular blood, intracerebral hematoma and high Fisher grade, clinical data as high Hunt and Hiss score, and laboratory data as sustained systemic inflammatory response. The rate of development of chronic hydrocephalus after aSAH was affected by treatment modality used to secure the ruptured aneurysm, whether microsurgical clipping or endovascular coiling, in some reports and not affected in other ones.[
It has been reported that maneuvers accelerating the clearance of blood in the basal cisterns could halt the development of symptomatic ventriculomegaly and shunt dependence after aSAH.[
MATERIALS AND METHODS
The present study is conducted with the approval of our university ethical committee. This is a retrospective analysis of prospectively collected data on patients with intracranial aneurysms (IAs) who were managed at our university emergency hospital between July 2013 and July 2019. We enrolled in this study patients with ruptured anterior circulation IAs who underwent microsurgical clipping of their ruptured IAs. Our plan was to assess the effect of performing EVD through a translamina terminalis tube inserted during clipping surgery on the development of chronic hydrocephalus after aSAH. We divided our patients into two groups; Group A: patients who underwent aneurysm clipping with lamina terminalis fenestration only without inserting EVD tube and Group B: patients in whom EVD through a translamina terminalis tube was done during clipping surgery. We excluded patients in whom EVD was done by conventional ventriculostomy through Kocher burr hole before surgery or through the Paine’s point during surgery and patients who presented by Hunt and Hess score V.
The general surgical steps for clipping of the ruptured IAs were essentially the same in both groups. Either lateral supraorbital or pterional craniotomy was done in all cases. After dural opening, CSF is released from the basal cisterns and from the lamina terminalis to lax the brain. The ruptured aneurysm is then clipped under temporary proximal control. In Group B, a ventriculostomy tube is inserted, under direct microscopic visualization, inside the third ventricle through the fenestrated lamina terminalis to come out beneath the basal brain surface [
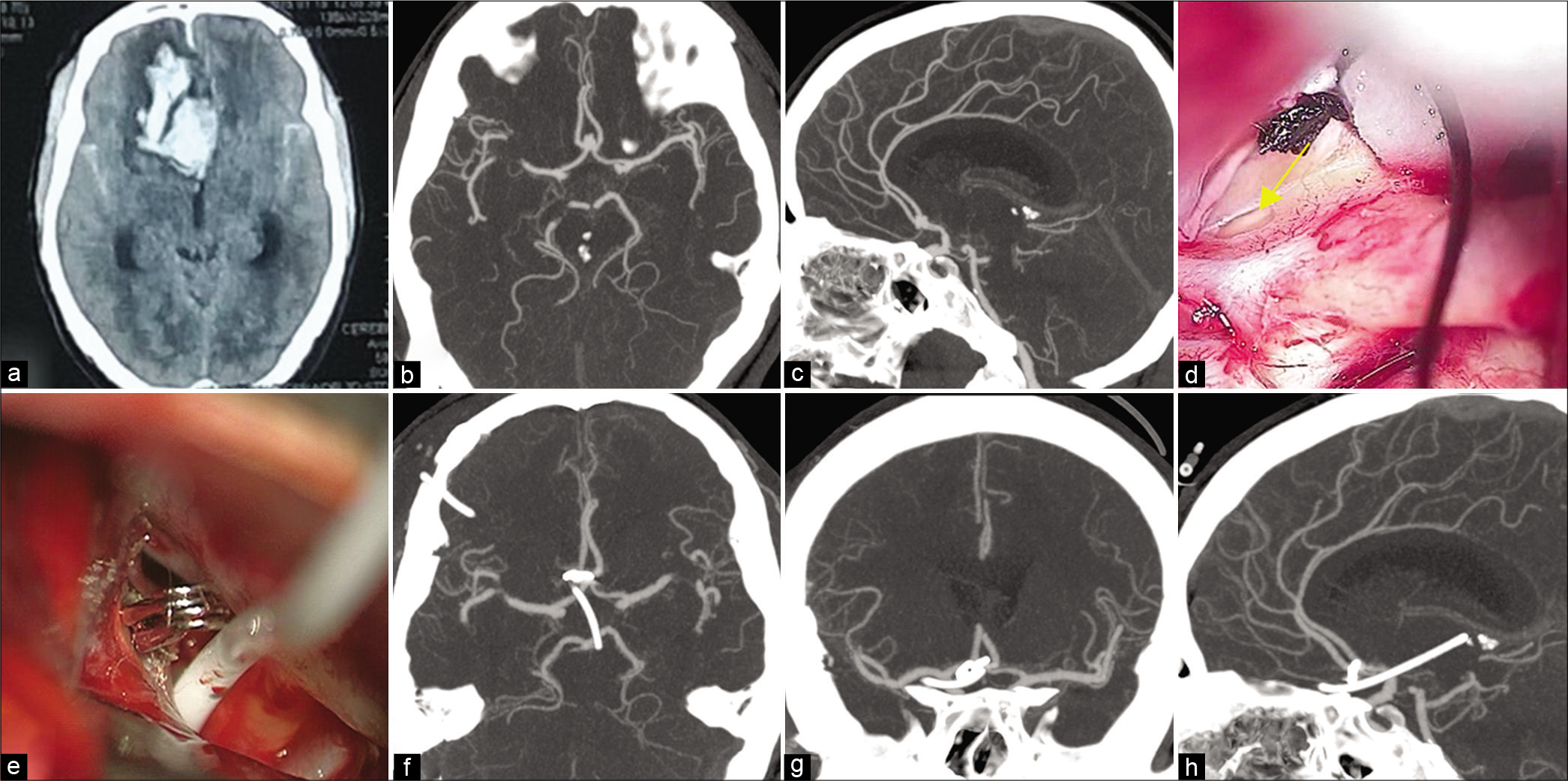
Figure 1:
(a) computed tomographic (CT) axial scan, obtained at admission, demonstrating subarachnoid hemorrhage (SAH) and intracerebral hematoma (ICH) in a 54-year-old woman with Hunt and Hess Grade IV. CT angiogram (b) axial view (c) sagittal view demonstrating a small aneurysm at the anterior communicating artery. (d) intraoperative microscopic image showing fenestrated lamina terminalis (arrow). (e) intraoperative microscopic image showing the catheter inserted inside the fenestrated lamina terminalis after clipping of the ruptured aneurysm. Postoperative CT angiogram (f) axial view (g) coronal view (h) sagittal view demonstrating the tip of the catheter in the third ventricle.
Statistical analysis
We assessed the distribution and frequency of all demographic, radiologic, and clinical variables. Covariates were compared between Group A and Group B using the Chi-squared test for categorical variables, the Student’s t-test for parametric continuous variables, and Mann–Whitney U-test for nonparametric continuous variables. P < 0.05 was considered statistically significant. All statistics were calculated using SPSS software version 25 (SPSS, Inc., Chicago, Illinois).
RESULTS
A total of 83 consecutive patients underwent microsurgical clipping for ruptured anterior circulation IAs at the Neurosurgery Department in Tanta University Hospitals, Egypt, between July 2013 and July 2019. Two of them had Hunt and Hess Grade V. Ten patients required insertion of ventriculostomy tube on admission before the definitive clipping surgery. In three patients, the intracranial pressure was extremely high and CSF release from basal cisterns and lamina terminalis was not possible except after inserting a ventriculostomy tube inside the lateral ventricle from Paine’s point. These 15 patients were excluded from our study. The remaining 68 patients were included in our study where lamina terminalis fenestration was done during clipping surgery without other ventriculostomy. We divided our patients into two groups; Group A operated on between July 2013 and June 2016 with lamina terminalis opening but without inserting ventriculostomy tube during clipping surgery and Group B patients operated on between July 2016 and July 2019 with lamina terminalis opening and with inserting a translamina terminalis EVD tube during the clipping surgery.
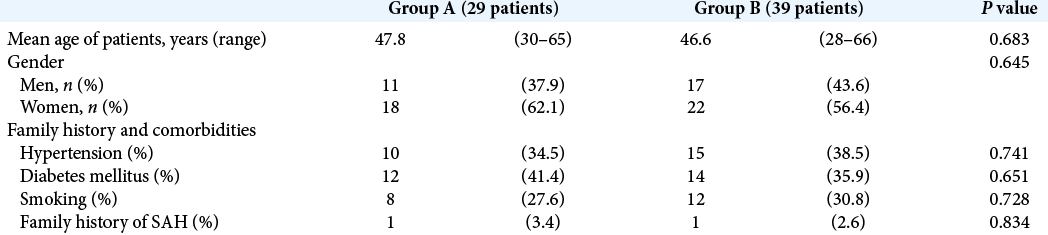
Demographic information for patients in both groups are summarized in [
Group A comprised 29 patients; 18 females and 11males, their age ranged from 30 to 65 years with a mean age of 47.8 years, 8 patients were smokers, 10 were hypertensive, 12 patients had diabetes mellitus, and 1 case had positive family history of aneurysmal subarachnoid hemorrhage [
On admission, 7 patients had H&H classification Grade II, 15 patients Grade III, and 7 patients Grade IV. Admission brain CT showed subarachnoid hemorrhage in all patients, hydrocephalus in 5 patients, intracerebral hematoma in 16 patients, and intraventricular hemorrhage in 14 patients. The location of the ruptured IA, in this group, was clearly defined by preoperative cerebral CTA. The ruptured aneurysm was located on the anterior communicating artery in 14 patients, on the MCA in 9 patients, the posterior communicating segment of ICA in 3 patients, on the ophthalmic segment of the ICA in 2 patients, and on the ICA bifurcation in 1 patient [
Most patients in this group underwent clipping surgery in the early period after aneurysm rupture. Seventeen patients were operated on within 24 h after aneurysm rupture, 7 patients in the 2nd day after rupture, and 5 patients were operated on late because of delayed referral and/or bad general condition at admission. In all patients, in this group, the lamina terminalis was fenestrated early in the operation with no associated complication [
During the early, the first 21 postoperative days, follow-up: nine patients had hydrocephalus and conventional VP shunt was inserted, two patients died (one from vasospasm and one from ventilator associated pneumonia), one patient had hemiparesis, and another patient was aphasic. Twenty-seven patients were scheduled for 6 months clinical and radiological follow-up. By the end of the 6 months follow-up period, two patients were lost, 7 patients developed hydrocephalus, and a ventriculoperitoneal shunt was inserted.
Group B comprised 39 patients; 22 females and 17 males, their age ranged from 28 to 66 years with a mean age of 46.6 years, 12 patients were smokers, 15 were hypertensive, 14 had diabetes mellitus, and 1 case had positive family history of aneurysmal subarachnoid hemorrhage [
On admission, 10 patients had H&H classification Grade II, 18 patients Grade III, and 11 patients Grade IV. Admission brain CT showed subarachnoid hemorrhage in all patients, hydrocephalus in 7 patients, intracerebral hematoma in 16 patients, and intraventricular hemorrhage in 22 patients. The location of the ruptured IA, in this group, was clearly defined by preoperative cerebral CTA. The ruptured aneurysm was located on the anterior communicating artery in 15 patients, on the MCA in 15 patients, the posterior communicating segment of ICA in 5 patients, on the ophthalmic segment of the ICA in 2 patients, and on the ICA bifurcation in 2 patients [
Most patients in this group underwent clipping surgery in the early period after aneurysm rupture. Twenty-six patients were operated on within 24 h after aneurysm rupture, 8 patients in the 2nd day after rupture, and 5 patients were operated on late because of delayed referral and/or bad general condition at admission. In all patients, in this group, a translamina terminalis, EVD tube was inserted regardless presence or absence of hydrocephalus. We encountered no complication related to lamina terminalis fenestration or tube insertion.
During the early, the first 21 postoperative days, follow- up: eight patients had positive CSF findings for infection during daily CSF analysis and were treated by systemic and intraventricular antibiotic injection. Infection subsided in all patients. Eight patients developed hydrocephalus; the tube was obstructed by blood clots in two patients and slipped in two patients. Three patients failed weaning with evidence of neurologic decline and ventriculomegaly during tube occlusion. One patient developed hydrocephalus and neurologic decline 2 days after tube removal in the 12th postoperative day. Conventional VP shunt was ultimately inserted for those eight patients. One patient had lower limb monoparesis, another patient had 3rd nerve palsy, and one patient was aphasic. Two patients died (one from cardiopulmonary problems and one from electrolyte disturbances). Thirty-seven patients were scheduled for 6 months clinical and radiological follow-up. By the end of the 6 months follow-up period, two patients were lost, four patients developed hydrocephalus, and a ventriculoperitoneal shunt was inserted.
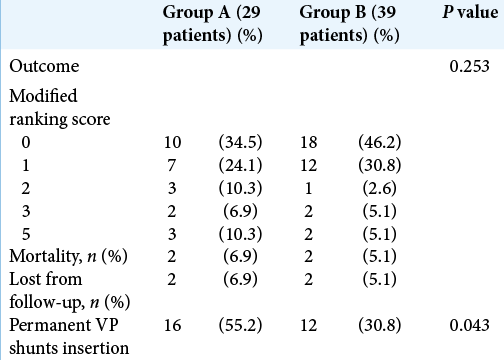
We used the modified Rankin score to document clinical outcome of our patients; Group A included 10 patients symptom free (Grade 0), seven patients Grade 1, three patients Grade 2, two patients Grade 3, and three patients Grade 5, while Group B included 18 patients Grade 0, 12 patients Grade 1, one patient Grade 2, two patients Grade 3, and two patients Grade 5. Two cases were lost from follow-up in each group and two cases have expired during their hospital stay in each group. Statistically, there was no significant difference in outcome of both groups. The incidence of permanent VP shunt dependence was significantly lower in Group B than in Group A patients (30.8% vs. 55.2%, respectively) [
DISCUSSION
Hydrocephalus is a common finding with aSAH and a major contributor to the occurrence of vasospasm with subsequent poor clinical outcomes.[
The lamina terminalis is a thin sheet of gray matter and pia mater stretches upward between the optic chiasm and rostrum of the corpus callosum. It forms the anterior wall of the third ventricle.[
The cornerstone in operating on ruptured IAs is to have slack brain for minimal brain retraction at the same time of maintaining adequate cerebral perfusion. In some situations of massive subarachnoid hemorrhage (SAH) or presence of a large space-occupying intracerebral hematoma with angry brain and awkward neuroanesthetic measures, CSF release is the way of choice to decrease the intracranial pressure and minimize brain retraction.[
We used to open the lamina terminalis in all cases of ruptured anterior circulation aneurysms treated through pterional or lateral supraorbital approaches. We believe this allows release of significant amount of CSF from the third ventricle rendering the brain lax and facilitating aneurysm dissection and clipping with minimal brain retraction. Furthermore, it is considered similar to endoscopic third ventriculostomy and helps in management of hydrocephalus in the acute stage. Since July 2016, we started to leave a silicon tube inside the third ventricle through the fenestrated lamina terminalis and connect to EVD system to wash bloody CSF from the ventricles to outside of the body aiming to ultimately decrease arachnoid scarring. We typically open basal cisterns around the carotid artery and optic nerves then fenestrate the lamina terminalis early before starting dissection of the aneurysm for clipping. In one case of ruptured anterior communicating aneurysm, the dome was projecting downward into the lamina terminalis. Hence, lamina terminalis fenestration was performed after securing the aneurysm. The lamina terminalis tube, when used, was always inserted after final clipping of the aneurysm not to obscure the operative field.
The technique of lamina terminalis ventriculostomy during microsurgery for ruptured IAs has provoked some criticism. It was claimed that a conventional ventriculostomy through Kocher burr hole before surgery or intraoperative ventriculostomy through the Paine’s point would be more reasonable to avoid retraction of swollen brain, especially when there is no need to approach the midline. The big advantage of lamina terminalis ventriculostomy is that it is done under direct vision which avoids all possible complications of other blind ventriculostomy techniques. We also avoid performing extra burr holes and the tube can be inserted safely and CSF drained regardless the size of the ventricle. Furthermore, frontal lobe is retracted for only short period till lamina terminalis is reached and there was no complication from this brief retraction.[
Lehto et al.[
The results of Lehto et al.[
In the literature, there is broad variation in the incidence of ventriculostomy-related infection ranging from 1% to 27%. There is also wide difference in management strategies. Among these strategies are the catheter exchange which cannot be simply done for the lamina terminalis ventriculostomy tube, while it can be simply done for conventional ventriculostomy tubes. The reported data do not justify this catheter exchange for infection prophylaxis as it does not reduce the risk of CSF infection.[
Cerebral CTA was our imaging modality of choice for diagnosis and surgical planning in this series. We believe that multislice helical CTA is more practical than digital subtraction angiography because it is highly sensitive, easily available at all times, less time consuming and is less invasive. CT images can easily demonstrate hydrocephalus, intracerebral hematoma, intraventricular hemorrhage, and even calcification in the vessel wall or the aneurysm. The 3D reconstruction helps to show the surgical view and aneurysm relation to bony landmarks as the anterior clinoid process.
There have been some features reported to be associated with increased incidence of development of chronic hydrocephalus after aSAH including acute stage hydrocephalus, poor Fisher’s grade, and poor Hunt and Hess grade.[
We had no significant difference (P > 0.05) between both groups regarding these factors which might influence the rate of development of chronic hydrocephalus [
Reviewing the literature regarding the histopathological basis[
Limitations
The results of the present work must be interpreted in the light of small number of cases and the retrospective nature of the study. Prospective studies with larger number of cases are recommended to overcome these limitations in forthcoming research.
CONCLUSION
EVD through fenestrated lamina terminalis is safe and may be effective in decreasing the incidence of chronic shunt- dependent hydrocephalus after aSAH.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adams H, Ban VS, Leinonen V, Aoun SG, Huttunen J, Saavalainen T. Risk of shunting after aneurysmal subarachnoid hemorrhage: A collaborative study and initiation of a consortium. Stroke. 2016. 47: 2488-96
2. Adil SM, Liu B, Charalambous LT, Kiyani M, Gramer R, Swisher CB. Healthcare economics of hydrocephalus after aneurysmal subarachnoid hemorrhage in the United States. Transl Stroke Res. 2019. 10: 650-63
3. Akyuz M, Tuncer R. The effects of fenestration of the interpeduncular cistern membrane arousted to the opening of lamina terminalis in patients with ruptured ACoA aneurysms: A prospective, comparative study. Acta Neurochir (Wien). 2006. 148: 725-3
4. Alleyne CH, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000. 47: 1124-7
5. Andaluz N, Zuccarello M. Fenestration of the lamina terminalis as a valuable adjunct in aneurysm surgery. Neurosurgery. 2004. 55: 1050-9
6. Arabi Y, Memish ZA, Balkhy HH, Francis C, Ferayan A, Al Shimemeri A. Ventriculostomy-associated infections: Incidence and risk factors. Am J Infect Control. 2005. 33: 137-43
7. Auer LM, Mokry M. Disturbed cerebrospinal fluid circulation after subarachnoid hemorrhage and acute aneurysm surgery. Neurosurgery. 1990. 26: 804-8
8. Aydin MD, Kanat A, Turkmenoglu ON, Yolas C, Gundogdu C, Aydin N. Changes in number of water-filled vesicles of choroid plexus in early and late phase of experimental rabbit subarachnoid hemorrhage model: The role of petrous ganglion of glossopharyngeal nerve. Acta Neurochir (Wien). 2014. 156: 1311-7
9. Black PM. Hydrocephalus and vasospasm after subarachnoid hemorrhage from ruptured intracranial aneurysms. Neurosurgery. 1986. 18: 12-6
10. Bota DP, Lefranc F, Vilallobos HR, Brimioulle S, Vincent JL. Ventriculostomy-related infections in critically ill patients: A 6-year experience. J Neurosurg. 2005. 103: 468-72
11. Chang SI, Tsai MD, Yen DH, Hsieh CT. The clinical predictors of shunt-dependent hydrocephalus following aneurysmal subarachnoid hemorrhage. Turk Neurosurg. 2018. 28: 36-42
12. Chohan MO, Carlson AP, Hart BL, Yonas H. Lack of functional patency of the lamina terminalis after fenestration following clipping of anterior circulation aneurysms. J Neurosurg. 2013. 119: 629-33
13. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American heart association/ American stroke association. Stroke. 2012. 43: 1711-37
14. Dasic D, Hanna SJ, Bojanic S, Kerr RS. External ventricular drain infection: The effect of a strict protocol on infection rates and a review of the literature. Br J Neurosurg. 2006. 20: 296-300
15. de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V. Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: A single-institution series and meta-analysis. Neurosurgery. 2007. 61: 924-33
16. Dehdashti AR, Rilliet B, Rufenacht DA, de Tribolet N. Shunt-dependent hydrocephalus after rupture of intracranial aneurysms: A prospective study of the influence of treatment modality. J Neurosurg. 2004. 101: 402-7
17. Di Russo P, Di Carlo DT, Lutenberg A, Morganti R, Evins AI, Perrini P. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. A systematic review and meta-analysis. J Neurosurg Sci. 2019. 64: 181-9
18. Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003. 52: 763-9
19. Erixon HO, Sorteberg A, Sorteberg W, Eide PK. Predictors of shunt dependency after aneurysmal subarachnoid hemorrhage: Results of a single-center clinical trial. Acta Neurochir (Wien). 2014. 156: 2059-69
20. Flibotte JJ, Lee KE, Koroshetz WJ, Rosand J, McDonald CT. Continuous antibiotic prophylaxis and cerebral spinal fluid infection in patients with intracranial pressure monitors. Neurocrit Care. 2004. 1: 61-8
21. Kanat A, Turkmenoglu O, Aydin MD, Yolas C, Aydin N, Gursan N. Toward changing of the pathophysiologic basis of acute hydrocephalus after subarachnoid hemorrhage: A preliminary experimental study. World Neurosurg. 2013. 80: 390-5
22. Komotar RJ, Olivi A, Rigamonti D, Tamargo RJ. Microsurgical fenestration of the lamina terminalis reduces the incidence of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002. 51: 1403-12
23. Koyanagi M, Fukuda H, Saiki M, Tsuji Y, Lo B, Kawasaki T. Effect of choice of treatment modality on the incidence of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2018. 130: 949-55
24. Lehto H, Dashti R, Karatas A, Niemela M, Hernesniemi JA. Third ventriculostomy through the fenestrated lamina terminalis during microneurosurgical clipping of intracranial aneurysms: An alternative to conventional ventriculostomy. Neurosurgery. 2009. 64: 430-4
25. Lo CH, Spelman D, Bailey M, Cooper DJ, Rosenfeld JV, Brecknell JE. External ventricular drain infections are independent of drain duration: An argument against elective revision. J Neurosurg. 2007. 106: 378-83
26. Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES Jr. Ventriculostomy-related infections: A critical review of the literature. Neurosurgery. 2002. 51: 170-81
27. Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001. 33: 2028-33
28. Maniker AH, Vaynman AY, Karimi RJ, Sabit AO, Holland B. Hemorrhagic complications of external ventricular drainage. Neurosurgery. 2006. 59: ONS419-24
29. Mao J, Zhu Q, Ma Y, Lan Q, Cheng Y, Liu G. Fenestration of lamina terminalis during anterior circulation aneurysm clipping on occurrence of shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: Meta-analysis. World Neurosurg. 2019. 129: e1-5
30. Mijderwijk HJ, Fischer I, Zhivotovskaya A, Bostelmann R, Steiger HJ, Cornelius JF. Prognostic model for chronic shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019. 124: e572-e579
31. Nakatsuka Y, Kawakita F, Yasuda R, Umeda Y, Toma N, Sakaida H. Preventive effects of cilostazol against the development of shunt-dependent hydrocephalus after subarachnoid hemorrhage. J Neurosurg. 2017. 127: 319-26
32. O’Kelly CJ, Kulkarni AV, Austin PC, Urbach D, Wallace MC. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: Incidence, predictors, and revision rates. Clinical article. J Neurosurg. 2009. 111: 1029-35
33. Paisan GM, Ding D, Starke RM, Crowley RW, Liu KC. Shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage: Predictors and long-term functional outcomes. Neurosurgery. 2018. 83: 393-402
34. Park P, Garton HJ, Kocan MJ, Thompson BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery. 2004. 55: 594-9
35. Pfisterer W, Muhlbauer M, Czech T, Reinprecht A. Early diagnosis of external ventricular drainage infection: Results of a prospective study. J Neurol Neurosurg Psychiatry. 2003. 74: 929-32
36. Pietila TA, Heimberger KC, Palleske H, Brock M. Influence of aneurysm location on the development of chronic hydrocephalus following SAH. Acta Neurochir (Wien). 1995. 137: 70-3
37. Randell T, Niemela M, Kytta J, Tanskanen P, Maattanen M, Karatas A. Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage: The Helsinki experience. Surg Neurol. 2006. 66: 382-8
38. Saliou G, Paradot G, Gondry C, Bouzerar R, Lehmann P, Meyers ME. A phase-contrast MRI study of acute and chronic hydrodynamic alterations after hydrocephalus induced by subarachnoid hemorrhage. J Neuroimaging. 2012. 22: 343-50
39. Sandalcioglu IE, Stolke D. Failure of regular external ventricular drain exchange to reduce CSF infection. J Neurol Neurosurg Psychiatry. 2003. 74: 1598-9
40. Schade RP, Schinkel J, Visser LG, Van Dijk JM, Voormolen JH, Kuijper EJ. Bacterial meningitis caused by the use of ventricular or lumbar cerebrospinal fluid catheters. J Neurosurg. 2005. 102: 229-34
41. Sheehan JP, Polin RS, Sheehan JM, Baskaya MK, Kassell NF. Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1999. 45: 1120-7
42. Sindou M. Favourable influence of opening the lamina terminalis and Lilliequist’s membrane on the outcome of ruptured intracranial aneurysms. A study of 197 consecutive cases. Acta Neurochir (Wien). 1994. 127: 15-6
43. Tapaninaho A, Hernesniemi J, Vapalahti M, Niskanen M, Kari A, Luukkonen M. Shunt-dependent hydrocephalus after subarachnoid haemorrhage and aneurysm surgery: Timing of surgery is not a risk factor. Acta Neurochir (Wien). 1993. 123: 118-24
44. Tomasello F, d’Avella D, de Divitiis O. Does lamina terminalis fenestration reduce the incidence of chronic hydrocephalus after subarachnoid hemorrhage?. Neurosurgery. 1999. 45: 827-31
45. Vale FL, Bradley EL, Fisher WS. The relationship of subarachnoid hemorrhage and the need for postoperative shunting. J Neurosurg. 1997. 86: 462-6
46. Wessell AP, Kole MJ, Cannarsa G, Oliver J, Jindal G, Miller T. A sustained systemic inflammatory response syndrome is associated with shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019. 130: 1984-91
47. Wilson CD, Safavi-Abbasi S, Sun H, Kalani MY, Zhao YD, Levitt MR. Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017. 126: 586-95
48. Wong GK, Poon WS, Wai S, Yu LM, Lyon D, Lam JM. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: Result of a randomised controlled trial. J Neurol Neurosurg Psychiatry. 2002. 73: 759-61
49. Yamada S, Ishikawa M, Yamamoto K, Ino T, Kimura T, Kobayashi S. Aneurysm location and clipping versus coiling for development of secondary normal-pressure hydrocephalus after aneurysmal subarachnoid hemorrhage: Japanese stroke data bank. J Neurosurg. 2015. 123: 1555-61
50. Yasargil MG.editors. Microneurosurgery, Volume I: Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms. Germany: Thieme; 1984. p.
51. Yolas C, Ozdemir NG, Kanat A, Aydin MD, Keles P, Kepoglu U. Uncovering a new cause of obstructive hydrocephalus following subarachnoid hemorrhage: Choroidal artery vasospasm-related ependymal cell degeneration and aqueductal stenosis-first experimental study. World Neurosurg. 2016. 90: 484-91
52. Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C. Efficacy of antimicrobial-impregnated external ventricular drain catheters: A prospective, randomized, controlled trial. J Neurosurg. 2003. 98: 725-30