- Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States,
- Department of Neurology, Baylor College of Medicine, Houston, Texas, United States.
Correspondence Address:
Adrish Anand, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
DOI:10.25259/SNI_241_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Adrish Anand1, Jay R. Gavvala2, Raissa Mathura1, Ricardo A. Najera1, Ron Gadot1, Ben Shofty1, Sameer A. Sheth1. Elimination of anxiety after laser interstitial thermal ablation of the dominant cingulate gyrus for epilepsy. 29-Apr-2022;13:178
How to cite this URL: Adrish Anand1, Jay R. Gavvala2, Raissa Mathura1, Ricardo A. Najera1, Ron Gadot1, Ben Shofty1, Sameer A. Sheth1. Elimination of anxiety after laser interstitial thermal ablation of the dominant cingulate gyrus for epilepsy. 29-Apr-2022;13:178. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11575
Abstract
Background: Anxiety is a common symptom of mental health disorders. Surgical treatment of anxiety-related disorders is limited by our understanding of the neural circuitry responsible for emotional regulation. Limbic regions communicate with other cortical and subcortical regions to generate emotional responses and behaviors toward anxiogenic stimuli. Epilepsy involving corticolimbic regions may disrupt normal neural circuitry and present with mood disorders. Anxiety presenting in patients with mesial temporal lobe epilepsy is common; however, anxiety in patients with cingulate epilepsy is not well described. Neurosurgical cases with rare clinical presentations may provide insight into the basic functionality of the human mind and ultimately lead to improvements in surgical treatments.
Case Description: We present the case of a 24-year-old male with a 20-year history of nonlesional and cingulate epilepsy with an aura of anxiety and baseline anxiety. Noninvasive work-up was discordant. Intracranial evaluation using stereoelectroencephalography established the epileptogenic zone in the left anterior and mid-cingulate gyrus. Stimulation of the cingulate reproduced a sense of anxiety typical of the habitual auras. We performed laser interstitial thermal therapy of the left anterior and mid-cingulate gyrus. At 8 months following ablation, the patient reported a substantial reduction in seizure frequency and complete elimination of his baseline anxiety and anxious auras.
Conclusion: This case highlights the role of the cingulate cortex (CC) in regulating anxiety. Ablation of the epileptic focus resolved both epilepsy-related anxiety and baseline features.a Future studies assessing the role of the CC in anxiety disorders may enable improvements in surgical treatments for anxiety disorders.
Keywords: Cingulate epilepsy, Epilepsy surgery, Ictal anxiety, Laser interstitial thermal therapy, Stereo-encephalography, Stereotactic laser ablation
INTRODUCTION
Anxiety is a debilitating symptom that occurs in 28% of adults and up to 60% of epilepsy patients.[
In epilepsy with foci located in corticolimbic structures, disruption of normal circuitry may result in anxiety presenting concurrently with epilepsy or as part of the seizure.[
Here, we describe a case of a young patient with nonlesional, focal, dominant cingulate epilepsy with strong anxious auras, and high baseline anxiety. We utilized stereoelectroencephalography (sEEG) and laser interstitial thermal therapy (LITT) to localize and ablate, respectively, epileptogenic foci in the anterior CC (ACC) and middle CC (MCC). Following surgical ablation, the patient reported complete elimination of baseline anxiety and anxious auras along with adequate seizure reduction. The patient’s clinical presentation, treatment outcome, and a discussion of the literature are presented.
CASE PRESENTATION
History and presentation
A 24-year-old right-handed man with no relevant medical history presented with a history of epilepsy since 4 years of age. Initially, his seizures occurred at night accompanied by bouts of crying and bad dreams. His semiology then progressed to seizures consisting of loss of balance, increased muscle tension, and “zoning out.” In the 3 years before epilepsy surgery, his seizure semiology changed and consisted of an aura of confusion and anxiety followed by an ictal period of arousal, body tingling, leg extension, and thrashing of the arms and legs lasting 30–50 s. Sometimes, the thrashing would progress to generalized bilateral tonic-clonic seizures, followed by postictal confusion. His seizures mainly occurred while asleep and occurred up to every other night.
Since turning 21, the patient’s seizures have become more frequent, and he reported increased anxiety associated with the seizures despite no clear life events that may have triggered the increase in anxiety. The patient reported avoiding situations or conversations that may cause distress, such as attending college courses or discussing his health condition. The patient took sertraline to manage his anxiety, which provided moderate relief of baseline anxiety but had no therapeutic effect on his anxious auras. His anxiety-induced avoidance behavior substantially impaired his quality of life.
Following his initial diagnosis, the patient was treated with oxcarbazepine and was seizure-free for 1 year between 6 and 7 years of age. After his seizures returned, the dosage of oxcarbazepine was increased without benefit. Topiramate, levetiracetam, zonisamide, and lacosamide were added to his antiepileptic drug regimen at various points but were discontinued due to side effects. Before surgery, the patient was on oxcarbazepine and cannabidiol for his epilepsy.
Phase 1 evaluation: Noninvasive work-up
The patient was admitted to the epilepsy monitoring unit for scalp EEG, during which he had six habitual seizures. EEG was nonlocalizing, as no clear ictal pattern was discerned. However, the clinical characteristics of nocturnal predominance, brief duration, maintained awareness, and hyperkinetic features suggested a mesial frontal epileptogenic zone. The patient’s head turning to the right suggested lateralization to the left hemisphere, but a “Figure of 4 sign” seen late in the generalized seizure with the left arm extended was suggestive of a right hemisphere source. Complex bilateral and hyperkinetic movements suggested a midline or supplementary motor area involvement. As there were no clear ictal EEG changes or interictal epileptiform discharges, deeper structures such as the mesial parietal or frontal areas were hypothesized to be involved. Observations of ictal tachycardia further suggested a mesial temporal source.
Brain magnetic resonance imaging (MRI) appeared normal. Positron emission tomography showed hypometabolism in the temporal lobes bilaterally, with a greater reduction in the right temporal lobe. Magnetoencephalography showed a single right-sided posterior insular cluster. Both magnetoencephalography and positron emission tomography findings were discordant with the semiology. Neuropsychiatric evaluation showed elevated baseline anxiety and poor distress tolerance.
The patient’s case was presented to a multidisciplinary epilepsy surgery conference. Given that the data obtained from the noninvasive work-up were non-conclusive, the clinical team decided that the patient would be a suitable candidate for intracranial evaluation using sEEG for further localization of the seizure focus.
Phase 2 evaluation: Stereoencephalography
The patient underwent a sEEG study with bilateral frontotemporal and parietal coverage including the insular cortex and CC [
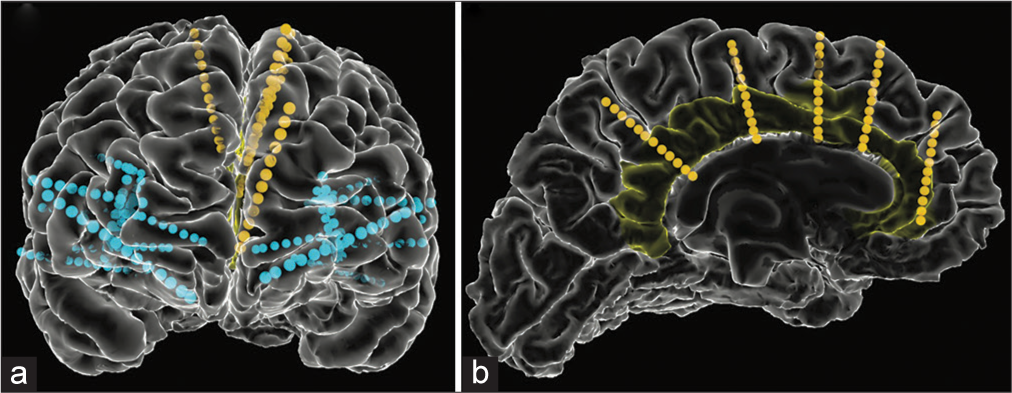
Figure 1:
Stereoencephalography electrode placement. (a) Anterior view of bilateral electrode placement in frontal and temporal lobes with insular and cingulate coverage. Yellow and blue lines represent electrodes targeting the cingulate gyrus and non-cingulate regions respectively. (b) Left cingulate electrode coverage with the cingulate gyrus highlighted in yellow.
Throughout his hospital stay, the patient expressed an overwhelming sense of anxiety regarding constant pain, headaches, and desires to leave the hospital. Notably, the severity of his anxiety increased as he was weaned off antiepileptic drugs to induce seizures.
LITT
After intracranial evaluation, the patient had nightly habitual seizures with a strong aura of anxiety. Based on Phases 1 and 2 evaluations, we presented the patient with laser ablation, surgical resection, responsive neural stimulation, or deep brain stimulation as treatment options. After deliberation, the patient chose to proceed with laser ablation. Roughly 3 months after his discharge from the intracranial evaluation, the patient underwent LITT ablation of his epileptic focus.
Under general anesthesia, the patient’s head was affixed to the ROSA using a Leksell (Elekta, Atlanta, GA, USA) frame, and fluoroscopic computed tomography (CT) was performed (O-arm O2, Medtronic, Minnesota, United States). The images were fused with the preoperative planning MRI and CT. The preoperative plan was loaded into the ROSA stereotactic robot and the robot was registered using the frame pins as skull fiducials. Our full stereotactic workflow has been described in detail.[
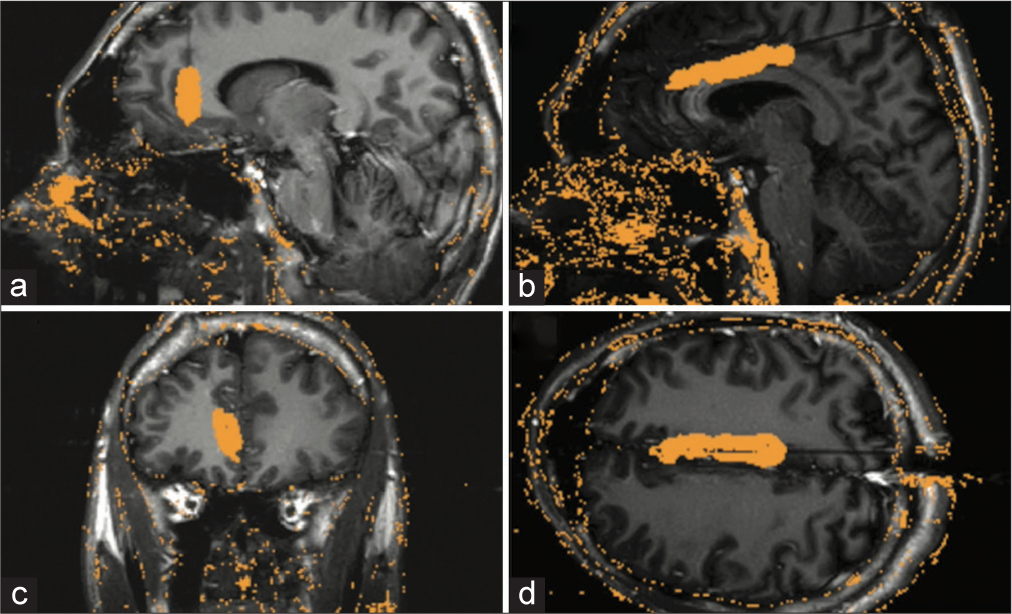
Figure 2:
Laser interstitial thermal therapy trajectory and ablation volume. (a) Sagittal view of the left anterior cingulate trajectory. (b) Coronal view of left anterior cingulate trajectory. (c) Sagittal view of the left middle cingulate trajectory. (d) Axial view of the left middle cingulate trajectory. Orange mask over the brain indicates the ablation volume of the trajectory.
Video 1
Postoperative follow-up
After surgery, the patient reported complete elimination of his anxiety. For 3 weeks after laser ablation, the patient experienced transient right upper and lower extremity weakness, congruent with ablation of the posterior MCC near the left supplemental motor area. After 4 months, the patient reported a constellation of nonhabitual seizures consisting of abnormal sensations starting in both feet moving up through the body. Anxiety was not present in the semiology of seizures after laser ablation. The patient had a dramatic reduction in seizure frequency from daily seizures to a total of four seizures since ablation. The patient’s outcome at 8 months was categorized as Engel Class IIB.
DISCUSSION
Anxiety is a debilitating symptom caused by disruption of complex interconnected neural networks responsible for stimulus and emotion processing and integration. Defining brain regions and networks responsible for anxiety are necessary for developing effective therapies. Neurosurgical cases like ours, much like historical, and accidental lesion cases, provide a unique opportunity for understanding fundamental neural mechanisms that underlie complex human behavior. In the present case, we determined that the CC was responsible for anxiety regulation. Two prior studies describe four patients with cingulate epilepsy presenting with similar semiology consisting of auras of anxiety.[
Neurological substrate of anxiety
The ACC plays a role in mood regulation and cognitive control.[
The basis of the ACC’s contribution to anxiety can be attributed to its role as a hub for communication among areas responsible for emotional regulation and goal-oriented cognitive control.[
In the present case, LITT of the ACC provided complete elimination of ictal and baseline elevated anxiety levels. On initial presentation, the relationship between the patient’s anxiety and epilepsy was not clear. Before sEEG, increased anxiety, reported by the patient as his epilepsy burden increased, was characterized as a common comorbidity of increased seizure frequency rather than temporally linked to his seizures. After surgical ablation, it was evident that his baseline anxiety could have been a result of ictal activity in the ACC without progression to motor seizures. Furthermore, elimination of anxious auras with development of new semiology without anxiety after ablation provides more evidence that the patient’s anxiety was temporally and spatially linked to neural activity in the ACC. This case corroborates prior neuromodulation studies and further characterizes the role of the ACC in regulating anxiety.
Current surgical treatments for anxiety disorders are limited by the heterogeneity of phenotypes of anxiety disorders and poor characterization of the underlying neural circuitry. Given the role of the ACC as a hub for communication within emotional regulation networks and the dramatic results seen in this case, the ACC may serve as a general target for anxiety treatment through neuromodulation or surgical ablation. Few studies have assessed the effects of ACC neuromodulation for anxiety disorders; however, the future work that better defines the role of the ACC in anxiety may lead to therapeutic breakthroughs.[
Limitations
Although we provide strong evidence for the role of the ACC in regulating anxiety through a lesional study, findings arising from a single patient may limit the generalizability of the phenomenon. Still, our case supports prior findings in the literature regarding this causal relationship, which should be further studied in larger series of patients with similar ailments.
CONCLUSION
We present the case of a 24-year-old man with a 20-year history of dominant hemisphere focal nonlesional cingulate epilepsy with elevated baseline anxiety and anxious auras. The patient underwent sEEG with successful localization of the epileptogenic zone followed by LITT to ablate seizure foci in the ACC and MCC. Surgical ablation eliminated the patient’s baseline anxiety and anxious auras and provided significant seizure reduction 8-months postoperatively. This case provides further evidence supporting the central role of the ACC in anxiety disorders.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
This work was supported by the McNair Foundation (SS) and Dana Foundation (SS).
Conflicts of interest
SAS is a consultant for Boston Scientific, Neuropace, Abbott, and Zimmer Biomet. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
[Video 1]-Available on:
Acknowledgments
We acknowledge the patient and his family, without whom this report would not be possible.
References
1. Alkawadri R, Gonzalez-Martinez J, Gaspard N, Alexopoulos AV. Propagation of seizures in a case of lesional mid-cingulate gyrus epilepsy studied by stereo-EEG. Epileptic Disord. 2016. 18: 418-25
2. Alkawadri R, Mickey BE, Madden CJ, Van Ness PC. Cingulate gyrus epilepsy: Clinical and behavioral aspects, with surgical outcomes. Arch Neurol. 2011. 68: 381-5
3. Alkawadri R, So NK, Van Ness PC, Alexopoulos AV. Cingulate epilepsy. JAMA Neurol. 2013. 70: 995-1002
4. Bartolomei F, Trébuchon A, Gavaret M, Régis J, Wendling F, Chauvel P. Acute alteration of emotional behaviour in epileptic seizures is related to transient desynchrony in emotion-regulation networks. Clin Neurophysiol. 2005. 116: 2473-9
5. Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol Med. 2013. 43: 897-910
6. Berlin HA, Hamilton H, Hollander E. Experimental therapeutics for refractory obsessive-compulsive disorder: Translational approaches and new somatic developments. Mt Sinai J Med. 2008. 75: 174-203
7. Beyenburg S, Mitchell AJ, Schmidt D, Elger CE, Reuber M. Anxiety in patients with epilepsy: Systematic review and suggestions for clinical management. Epilepsy Behav. 2005. 7: 161-71
8. Bijanki KR, Manns JR, Inman CS, Choi KS, Harati S, Pedersen NP. Cingulum stimulation enhances positive affect and anxiolysis to facilitate awake craniotomy. J Clin Invest. 2019. 129: 1152-66
9. Boulogne S, Catenoix H, Ryvlin P, Rheims S. Long-lasting seizure-related anxiety in patients with temporal lobe epilepsy and comorbid psychiatric disorders. Epileptic Disord. 2015. 17: 340-4
10. Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015. 18: 1394-404
11. Chou CC, Lee CC, Lin CF, Chen YH, Peng SJ, Hsiao FJ. Cingulate gyrus epilepsy: Semiology, invasive EEG, and surgical approaches. Neurosurg Focus. 2020. 48: E8
12. Fossati P. Neural correlates of emotion processing: From emotional to social brain. Eur Neuropsychopharmacol. 2012. 22: S487-91
13. Fried I, Fahoum F, Frew A, Andelman F, Andelman-Gur MM, Salamon N. Laser ablation of human guilt. Brain Stimulat. 2022. 15: 164-6
14. Hartley CA, Phelps EA. Anxiety and decision-making. Biol Psychiatry. 2012. 72: 113-8
15. Hescham S, Jahanshahi A, Meriaux C, Lim LW, Blokland A, Temel Y. Behavioral effects of deep brain stimulation of different areas of the Papez circuit on memory-and anxiety-related functions. Behav Brain Res. 2015. 292: 353-60
16. Hingray C, McGonigal A, Kotwas I, Micoulaud-Franchi JA. The relationship between epilepsy and anxiety disorders. Curr Psychiatry Rep. 2019. 21: 40
17. Hoffman EJ, Mathew SJ. Anxiety disorders: A comprehensive review of pharmacotherapies. Mt Sinai J Med. 2008. 75: 248-62
18. Karas PJ, Giridharan N, Treiber JM, Prablek MA, Khan AB, Shofty B. Accuracy and workflow improvements for responsive neurostimulation hippocampal depth electrode placement using robotic stereotaxy. Front Neurol. 2020. 11: 590825
19. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005. 62: 593-602
20. Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005. 62: 617-27
21. Kwon OY, Park SP. Depression and anxiety in people with epilepsy. J Clin Neurol Seoul Korea. 2014. 10: 175-88
22. Lebow MA, Chen A. Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 2016. 21: 450-63
23. López-Gómez M, Espinola M, Ramirez-Bermudez J, Martinez-Juarez IE, Sosa AL. Clinical presentation of anxiety among patients with epilepsy. Neuropsychiatr Dis Treat. 2008. 4: 1235-9
24. McGovern RA, Sheth SA. Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: Converging evidence from cognitive neuroscience and psychiatric neurosurgery. J Neurosurg. 2017. 126: 132-47
25. Melo HM, Guarnieri R, Vascouto HD, Formolo DA, de Carvalho CR, Campos WK. Ictal fear is associated with anxiety symptoms and interictal dysphoric disorder in drug-resistant mesial temporal lobe epilepsy. Epilepsy Behav. 2021. 115: 107548
26. Pepper J, Hariz M, Zrinzo L. Deep brain stimulation versus anterior capsulotomy for obsessive-compulsive disorder: A review of the literature. J Neurosurg. 2015. 122: 1028-37
27. Powell R, Elwes R, Hamandi K, Mullatti N. Cingulate gyrus epilepsy. Pract Neurol. 2018. 18: 447-54
28. Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011. 12: 154-67
29. Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O. Anterior insula responds to temporally unpredictable aversiveness: An fMRI study. Neuroreport. 2014. 25: 596-600
30. Sheth SA, Neal J, Tangherlini F, Mian MK, Gentil A, Cosgrove GR. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: A prospective long-term follow-up of 64 patients. J Neurosurg. 2013. 118: 491-7
31. Sperli F, Spinelli L, Pollo C, Seeck M. Contralateral smile and laughter, but no mirth, induced by electrical stimulation of the cingulate cortex. Epilepsia. 2006. 47: 440-3
32. Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007. 164: 318-27
33. von Lehe M, Wagner J, Wellmer J, Clusmann H, Kral T. Epilepsy surgery of the cingulate gyrus and the frontomesial cortex. Neurosurgery. 2012. 70: 900-10
34. Wscieklica T, Silva MS, Lemes JA, Melo-Thomas L, Céspedes IC, Viana MB. Deep brain stimulation of the dorsal raphe inhibits avoidance and escape reactions and activates forebrain regions related to the modulation of anxiety/panic. Behav Brain Res. 2017. 321: 193-200