- Department of Neurosurgery Tsukuba Medical Center Hospital, Tsukuba, Japan.
- Department of Radiology, Tsukuba Medical Center Hospital, Tsukuba, Japan.
- Department of Neurosurgery Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
- Department of Stroke, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
Correspondence Address:
Go Ikeda
Department of Stroke, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
DOI:10.25259/SNI_806_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Toshihide Takahashi1, Go Ikeda1, Haruki Igarashi1, Takahiro Konishi2, Kota Araki1, Kei Hara1, Ken Akimoto1, Satoshi Miyamoto1, Masanari Shiigai2, Kazuya Uemura1, Eiichi Ishikawa3, Yuji Matsumaru4. Emergent carotid artery stenting for cervical internal carotid artery injury during carotid endarterectomy: A case report. 17-Mar-2021;12:109
How to cite this URL: Toshihide Takahashi1, Go Ikeda1, Haruki Igarashi1, Takahiro Konishi2, Kota Araki1, Kei Hara1, Ken Akimoto1, Satoshi Miyamoto1, Masanari Shiigai2, Kazuya Uemura1, Eiichi Ishikawa3, Yuji Matsumaru4. Emergent carotid artery stenting for cervical internal carotid artery injury during carotid endarterectomy: A case report. 17-Mar-2021;12:109. Available from: https://surgicalneurologyint.com/surgicalint-articles/10643/
Abstract
Background: Carotid endarterectomy (CEA) has been the standard preventive procedure for cerebral infarction due to cervical internal carotid artery stenosis, and internal shunt insertion during CEA is widely accepted. However, troubleshooting knowledge is essential because potentially life-threatening complications can occur. Herein, we report a case of cervical internal carotid artery injury caused by the insertion of a shunt device during CEA.
Case Description: A 78-year-old man with a history of hypertension, diabetes, and hyperuricemia developed temporary left hemiplegia. A former physician had diagnosed the patient with a transient cerebral ischemic attack. The patient’s medical history was significant for the right internal carotid artery stenosis, which was severe due to a vulnerable plaque. We performed CEA to remove the plaque; however, there was active bleeding in the distal carotid artery of the cervical region after we removed the shunt tube. Hemostasis was achieved through compression using a cotton piece. Intraoperative digital subtraction angiography (DSA) revealed severe stenosis at the internal carotid artery distal to the injury site due to hematoma compression. The patient underwent urgent carotid artery stenting and had two carotid artery stents superimposed on the injury site. On DSA, extravascular pooling of contrast media decreased on postoperative day (POD) 1 and then disappeared on POD 14. The patient was discharged home without sequela on POD 21.
Conclusion: In the case of cervical internal carotid artery injury during CEA, hemostasis can be achieved by superimposing a carotid artery stent on the injury site, which is considered an acceptable troubleshooting technique.
Keywords: Carotid endarterectomy, Emergent carotid artery stenting, Iatrogenic vessel injury
INTRODUCTION
Carotid endarterectomy (CEA) has been the standard preventive procedure for cerebral infarction due to cervical internal carotid artery stenosis.[
Herein, we report a case of vessel injury in the distal carotid artery of the cervical region caused by the insertion of a shunt device during CEA. Hence, we performed emergency carotid artery stenting (CAS), and the patient had a good outcome.
CASE DESCRIPTION
A 78-year-old man referred to our hospital had a history of hypertension, Type 2 diabetes, hyperuricemia, and chronic subdural hematoma. The patient visited a former physician due to transient ischemic attack causing paralysis on the left side. Cervical magnetic resonance angiography revealed severe stenosis of the right internal carotid artery. The patient did not experience impaired consciousness or neurologic deficits, and the coagulation/fibrinolytic testing results were not remarkable. A carotid echography revealed that the peak systolic velocity of the right internal carotid artery was 276 cm/s. The plaque was mobile with low echogenicity.
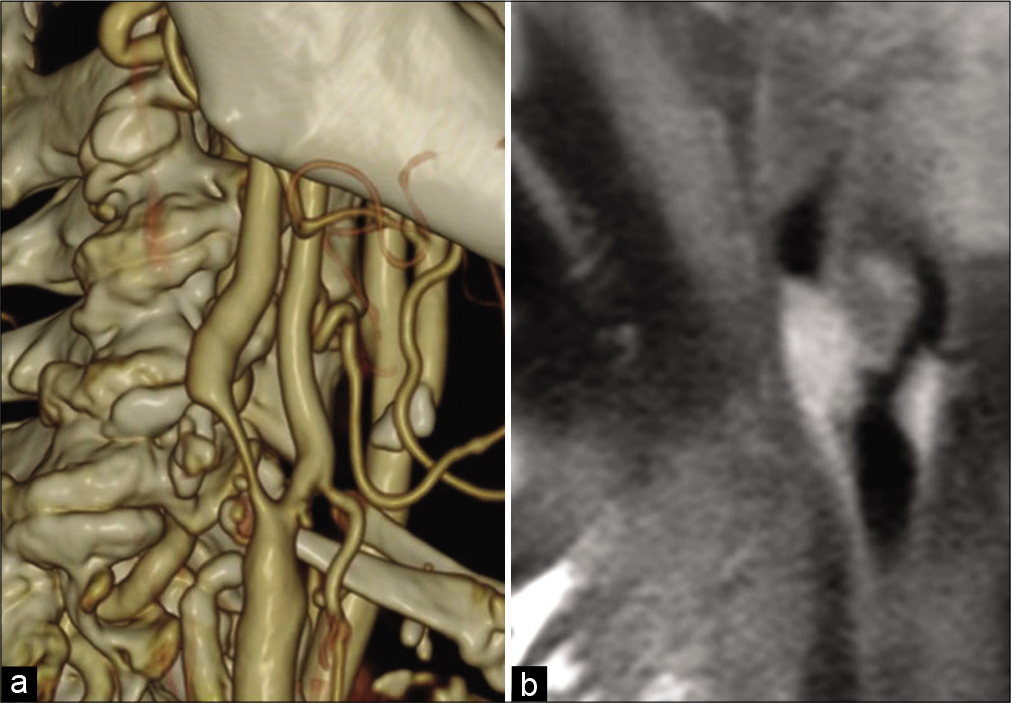
A cervical three-dimensional computed tomography angiography examination showed stenosis of the right carotid artery from C4/5 to C5/6. The stenosis rate was 71% based on the method used in the North American Symptomatic CEA Trial [
Figure 1:
Preoperative image. (a) Cervical three-dimensional computed tomography angiography: stenosis of the right carotid artery occurred from C4/5 to C5/6, and the stenosis rate was 71% based on the method used in the North American Symptomatic Carotid Endarterectomy Trial, (b) cervical magnetic resonance imaging: examination revealed an unstable plaque with a major axis of 20 mm showing T1 (black blood) high intensity from the right common carotid artery to the proximal internal carotid artery.
Cervical magnetic resonance imaging (MRI) revealed an unstable plaque with a major axis of 20 mm showing T1 (black blood) high intensity from the right common carotid artery to the proximal internal carotid artery [
The patient had severe symptomatic stenosis of the right internal carotid artery, which was an indication for revascularization. We planned to perform CEA because the plaque was vulnerable, and the patient did not present with high-risk factors for the procedure. Clopidogrel 75 mg/day was initiated at the time of onset but was discontinued 5 days before surgery.
We performed CEA in a hybrid operating room. The Furui’s double-balloon shunt system (Muranaka Medical Instruments, Osaka, Japan) was used, and balloons in both the common carotid artery side and the internal carotid artery side were injected with 0.5 mL of air. Then, we removed the plaque and sutured the carotid artery. An intraoperative digital subtraction angiography (DSA) revealed extravascular pooling of contrast media in the distal carotid artery of the cervical region, indicating vessel injury. The internal carotid artery, distal to the injury site, presented with severe stenosis due to hematoma compression [
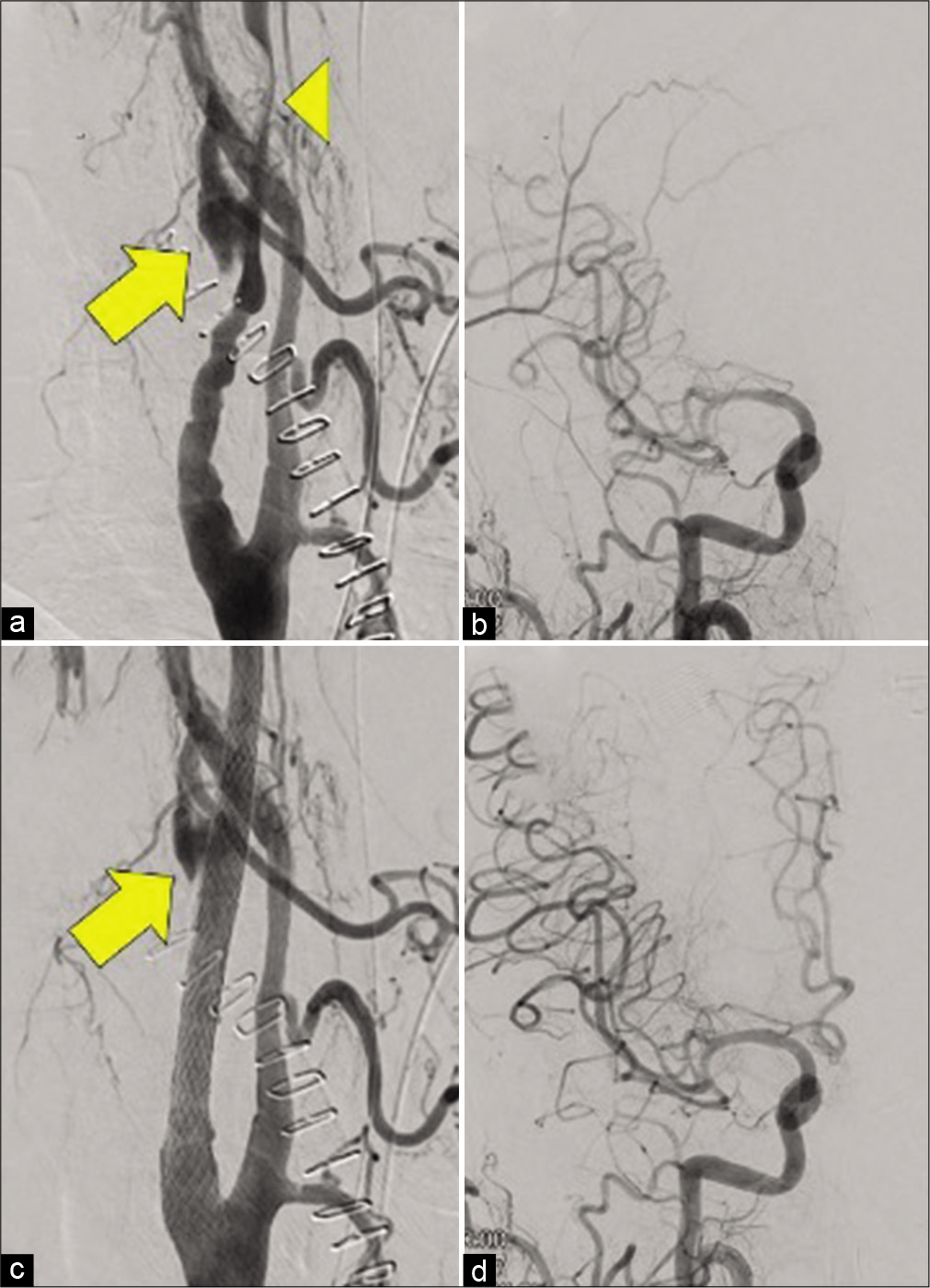
Figure 2:
Right common carotid angiography before and after carotid artery stenting (CAS). (a) Cervical angiography before CAS. Contrast media leaked from the injury site of the internal carotid artery (arrow), and the internal carotid artery distal to the injury point was severely stenotic due to hematoma compression (head arrow), (b) head angiography before CAS. Blood flow in the right middle cerebral artery was delayed, and the right anterior cerebral artery could not be visualized, (c) cervical angiography after CAS. The leakage of the contrast medium from the injury site decreased (arrow), (d) head angiography after CAS. Blood flow delay in the right middle cerebral artery improved, and the right anterior cerebral artery could be visualized.
Since we performed CEA in the hybrid operating room, immediately after the end of CEA, we also performed DSA with the transfemoral technique on the same bed. A right common carotid angiography revealed extravasation of the right internal carotid artery at the injury site, and contrast medium pooled in the extravascular space. The internal carotid artery, distal to the injury site, had severe stenosis and blood flow delay in the right middle cerebral artery. The right anterior cerebral artery could not be visualized [
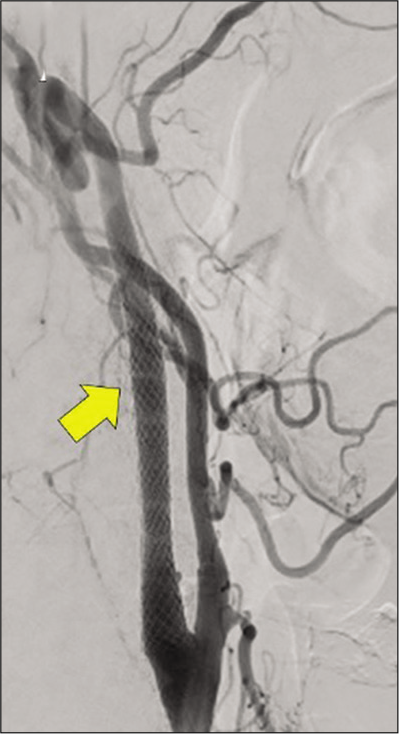
The Carotid WALLSTENT (Boston Scientific, Natick, MA, USA) measuring 8 × 29 mm was placed to cover the distal stenosis and the injury site. Although stenosis improved, blood flow delay from the injury site to the hematoma cavity remained. Hence, the Carotid WALLSTENT, measuring 10 × 31 mm, was placed and overlapped. Then, leakage of contrast medium from the injury site decreased [
Postoperatively, the patient continually received conservative management, including strict blood pressure control, under general anesthesia with intubation. Then, aspirin 100 mg and clopidogrel 75 mg were continuously administered. On DSA, extravascular pooling was further reduced at the end of CAS on postoperative day (POD) 1 [
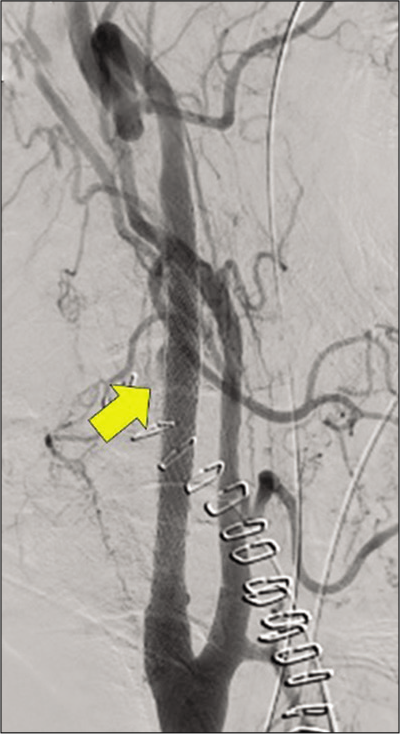
The patient was extubated on POD 6. He presented with hoarseness and right miosis due to Horner’s syndrome, that is, lower cranial nerve symptoms. However, both spontaneously disappeared overtime. Therefore, hematoma decompression was considered effective. A head MRI on POD 13 revealed no new cerebral infarction or intracranial hemorrhage. On DSA, the extravascular pooling of contrast media in the injury site disappeared on POD 14 [
Figure 5:
Right common carotid arteriography on postoperative day 14. The arrow indicates the point where the contrast media leaked due to injury as shown in
DISCUSSION
Inserting a shunt intraoperatively to prevent cerebral ischemia during CEA is widely accepted.[
In this case, the internal carotid injury site is more distal than the operative field, and it is easy to think of injury due to the physical stimulation of the shunt balloon. The volume of air used in balloon shunting is usually ≤0.3 mL, but we used 0.5 mL of air. Hence, overinflation cannot be denied. The shunt balloon device was moved due to surgery in the shunt system during plaque removal, and the balloon physically damaged the blood vessel.
There have been few reports on internal carotid artery injury due to shunt insertion. However, there is only one case on, which was reported by Illuminati et al. In that case, immediately after shunt insertion, the internal carotid artery was injured and bled.[
Some studies have shown that using a covered stent or a flow diverter stent for internal carotid artery injury had good outcomes. Arai et al. revealed that hemostasis was achieved by placing a covered stent for massive hemorrhage due to carotid artery erosion in esophageal cancer.[
Hagihara et al. showed that carotid artery stent placement for internal carotid artery injury prevented stroke in two cases.[
The flow diverting effect of the low wall coverage stent is controversial.[
The Japanese government has approved the use of carotid stents, such as the closed-cell type WALLSTENT, open-cell type Precise (Cardinal Health Inc., Dublin, Ireland), and Protégé (Medtronic, Dublin, Ireland). The coverage ratios are 21.4% for the closed-cell type WALLSTENT, 19.4% for the open-cell type Precise, and 17.6% for the Protégé stent.[
The coverage ratio of Pipeline (Medtronic, Dublin, Ireland), which is a flow diverter stent, is approximately 30%.[
There are some technical points of caution when a stent is placed in the carotid artery immediately after CEA. First, it was expected that hemostasis would be difficult with the placement of only one stent, and the distal position of the stent was determined assuming superposition from the beginning. Second, administration of antiplatelet drugs is controversial, but we thought that it would be better to administer them because of the possibility of stent superposition. Third, at the time of lesion crossing, multiple working angles created by three-dimensional rotational angiography were used to ensure crossing the true lumen safely.
Troubleshooting techniques for vessel injury during CEA include using covered stents and flow diverter stents. However, their availability is low in emergency settings. If such devices are difficult to obtain, an emergency procedure that can achieve hemostasis by superimposing carotid artery stents is worth trying.
CONCLUSION
In this case, CAS was performed urgently for a cervical internal carotid artery injury during CEA with a favorable outcome. Hemostasis can be achieved by superimposing a carotid artery stent on the injury site, which is considered an acceptable troubleshooting technique.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
1. Arai N, Kagami H, Funabiki T, Mine Y, Inaba M. Successful use of covered stent for carotid artery injury with active medial projecting extravasation. World Neurosurg. 2018. 112: 53-6
2. Assali AR, Sdringola S, Moustapha A, Rihner M, Denktas AE, Lefkowitz MA. Endovascular repair of traumatic pseudoaneurysm by uncovered self-expandable stenting with or without transstent coiling of the aneurysm cavity. Catheter Cardiovasc Interv. 2001. 53: 253-8
3. Barnett HJ, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991. 325: 445-53
4. . Executive committee for the asymptomatic carotid atherosclerosis study. JAMA. 1995. 273: 1421-8
5. Fargen KM, Hoh BL, Welch BG, Pride GL, Lanzino G, Boulos AS. Long-term results of enterprise stent-assisted coiling of cerebral aneurysms. Neurosurgery. 2012. 71: 239-44
6. Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC. The North American symptomatic carotid endarterectomy trial: Surgical results in 1415 patients. Stroke. 1999. 30: 1751-8
7. Giorgianni A, Pozzi F, Pellegrino C, Padovan S, Karligkiotis A, Castelnuovo P. Emergency placement of a flow diverter stent for an iatrogenic internal carotid artery injury during endoscopic pituitary surgery. World Neurosurg. 2019. 122: 376-9
8. Hagihara Y, Ueno M, Yamamoto A, Mizushima Y, Ishikawa K, Matsuoka T. Blunt cerebrovascular injury and its therapeutic strategy: Importance and efficiency of endovascular therapy. JJAAM. 2009. 20: 212-20
9. Illuminati G, Caliò FG, Pizzardi G, Vietri F. Internal carotid artery rupture caused by carotid shunt insertion. Int J Surg Case Rep. 2015. 14: 89-91
10. Kuramoto Y, Sakai N, Imamura H, Koyanagi M, Sakai C, Kunieda T. Endovascular therapy for cervical carotid artery aneurysms: Report of 4 cases. Surg Cereb Stroke. 2011. 39: 48-53
11. Lee JI, Ko JK, Lee TH, Choi CH, Lee SW, Cho WH. Sole stenting technique for the treatment of uncoilable very small aneurysms in the intracranial internal carotid artery. Neurol Med Chir (Tokyo). 2013. 53: 310-7
12. Li C, Li Y, Jiang C, Wu Z, Wang Y, Yang X. Stent alone treatment for dissections and dissecting aneurysms involving the basilar artery. J Neurointerv Surg. 2015. 7: 50-5
13. Liu H, Choe J, Jung SC, Song Y, Yang KH, Park KJ. Does a low-wall coverage stent have a flow diverting effect in small aneurysms?. Neurointervention. 2015. 10: 89-93
14. Lu J, Liu JC, Wang LJ, Qi P, Wang DM. Tiny intracranial aneurysms: Endovascular treatment by coil embolisation or sole stent deployment. Eur J Radiol. 2012. 81: 1276-81
15. Müller-Hülsbeck S, Schäfer PJ, Charalambous N, Schaffner SR, Heller M, Jahnke T. Comparison of carotid stents: An in vitro experiment focusing on stent design. J Endovasc Ther. 2009. 16: 168-77
16. Rerkasem K, Rothwell PM. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev. 2009. 2009: Cd000190
17. Santillan A, Greenberg E, Patsalides A, Salvaggio K, Riina HA, Govin YP. Long-term clinical and angiographic results of neuroform stent-assisted coil embolization in wide-necked intracranial aneurysms. Neurosurgery. 2012. 70: 1232-7
18. Sundt TM, Ebersold MJ, Sharbrough FW, Piepgras DG, Marsh WR, Messick JM. The risk-benefit ratio of intraoperative shunting during carotid endarterectomy. Relevancy to operative and postoperative results and complications. Ann Surg. 1986. 203: 196-204
19. Tamaki T, Yoji N, Saito N. Distal cervical carotid artery dissection after carotid endarterectomy: A complication of indwelling shunt. Int J Vasc Med. 2010. 2010: 816937
20. Troisi N, Dorigo W, Pulli R, Pratesi C. A case of traumatic internal carotid artery aneurysm secondary to carotid shunting. J Vasc Surg. 2010. 51: 225-7
21. Wang C, Tian Z, Liu J, Jing L, Paliwal N, Wang S. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: A comparison with enterprise stents and the pipeline device. J Transl Med. 2016. 14: 199
22. Wang K, Huang Q, Hong B, Li Z, Fang X, Liu J. Correlation of aneurysm occlusion with actual metal coverage at neck after implantation of flow-diverting stent in rabbit models. Neuroradiology. 2012. 54: 607-13
23. Wissgott C, Schmidt W, Behrens P, Brandt C, Schmitz KP, Andresen R. Experimental investigation of modern and established carotid stents. Rofo. 2014. 186: 157-65