- Department of Neurosurgery, Faculty of Medicine, Tanta University, Tanta, Gharbia, Egypt.
Correspondence Address:
Sherif Elsayed Elkheshin, Department of Neurosurgery, Faculty of Medicine, Tanta University, Tanta, Gharbia, Egypt.
DOI:10.25259/SNI_608_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sherif Elsayed Elkheshin, Mohamed Bebars. Endoscopic treatment of complex multiloculated hydrocephalus in children, steps that may help to decrease revision rate. 30-Aug-2021;12:434
How to cite this URL: Sherif Elsayed Elkheshin, Mohamed Bebars. Endoscopic treatment of complex multiloculated hydrocephalus in children, steps that may help to decrease revision rate. 30-Aug-2021;12:434. Available from: https://surgicalneurologyint.com/surgicalint-articles/11075/
Abstract
Background: Multiloculated hydrocephalus (MLH) is associated with increased intracranial pressure, with intraventricular septations, loculations, and isolation of parts of the ventricular system. Search continues for ideal surgical remedy capable of addressing the dimensions of the problem. We aimed to evaluate endoscopic septal fenestration and pellucidotomy combined with proximal shunt tube refashioning and further advancement into isolated loculations of the ventricular system containing choroid plexus.
Methods: This retrospective study was conducted on 55 patients with symptomatic complex MLH who underwent endoscopic surgery. The collected data included patients’ age, gender, presenting manifestations, operative details, rate of remission of preoperative clinical and imaging signs, postoperative complications, redo surgery, or extra shunt hardware insertion. Patients were divided into Group A (underwent the standard technique of endoscopic multiseptal wide fenestration and final ventriculoperitoneal shunt insertion) and Group B (modified technique by adding extra side ports along the proximal shunt hardware).
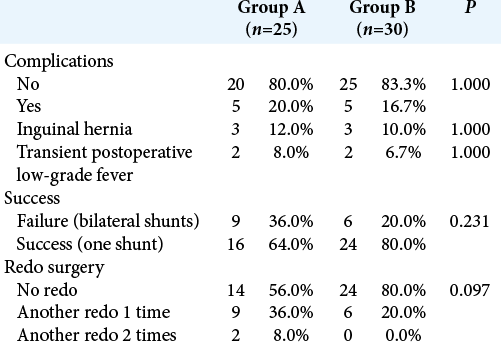
Results: Groups A and B included 25 and 30 patients, respectively. The percentage of patients showing improvement of almost all manifestations was higher in Group B compared to Group A, with no significant difference (P > 0.05). Group B had lower rate of complications (20% vs. 36%, P = 0.231), insertion of two shunts (16.7% vs. 20%, P = 1.000), and redo surgery (20% vs. 44%, P = 0.097).
Conclusion: The modified technique was associated with better outcomes in terms of the use of single shunt and redo surgery. Launching randomized clinical trials to compare the two techniques are recommended to ascertain the efficacy of the modified technique.
Keywords: Multiloculated hydrocephalus, Neuroendoscopy, Reoperation, Ventriculostomy
INTRODUCTION
Complex multiloculated hydrocephalus (MLH) is a clinical nonuniform ventriculomegaly associated with increased intracranial pressure mostly preceded by meningitis, ventriculitis, shunt infection, or intraventricular hemorrhage. This results in intraventricular septations, loculations, and isolation of parts of the ventricular system. Most neurosurgeons seek ideal surgical remedy capable of addressing the dimensions of problems. The surgical goals are normalization of the intracranial pressure using the least set of shunt hardware and minimization of redo and/or revision surgeries.[
Both microsurgical and endoscopic septal fenestration techniques have been described to serve the aforementioned surgical goals.[
The present study was conducted to evaluate the surgeons’ proposed effect of endoscopic septal fenestration and pellucidotomy combined with proximal shunt tube refashioning by adding extra side ports all around and further advancement into isolated loculations of the ventricular system containing choroid plexus. Further evaluation of clinical remission rate, keeping patient on single proximal shunt tube and the rate of revision and/or redo were also parts of the core of the study.
MATERIALS AND METHODS
Study design, settings, and ethical considerations
This retrospective cohort study was conducted on 55 patients presenting with symptomatic complex MLH and operated through pure endoscopic approach during October 1, 2017, through January 31, 2021. Ethical approval was obtained from the Institutional Review Board, Faculty of Medicine, Tanta University. Confidentiality of the patients’ data was maintained by assigning code numbers to patients that were known only by the researchers.
Eligibility criteria
The present study included patients with symptomatic complex MLH for whom endoscopic septal fenestration (including pellucidotomy) was performed with ventriculoperitoneal shunt inserted. The indication for surgery was based on clinical signs of increased intracranial pressure. Both computerized tomography and magnetic resonance imaging (1.5 Tesla) of the brain were performed for all patients.
Data collection
The patients’ data were thoroughly reviewed. The collected data included the patient’s age, gender, and presenting manifestations including head enlargement, dilated scalp veins, tense fontanel, delayed milestones, vomiting, papilledema, disturbed consciousness, weakness, and seizures. In addition, we recorded the postoperative details including the rate of remission of both preoperative clinical and imaging signs and complications. Any redo surgery or extra shunt hardware insertion was collected per case. Data of follow-up at 1, 6, and 12 months postoperatively were collected as regard any symptomatic recurrence.
Surgical technique
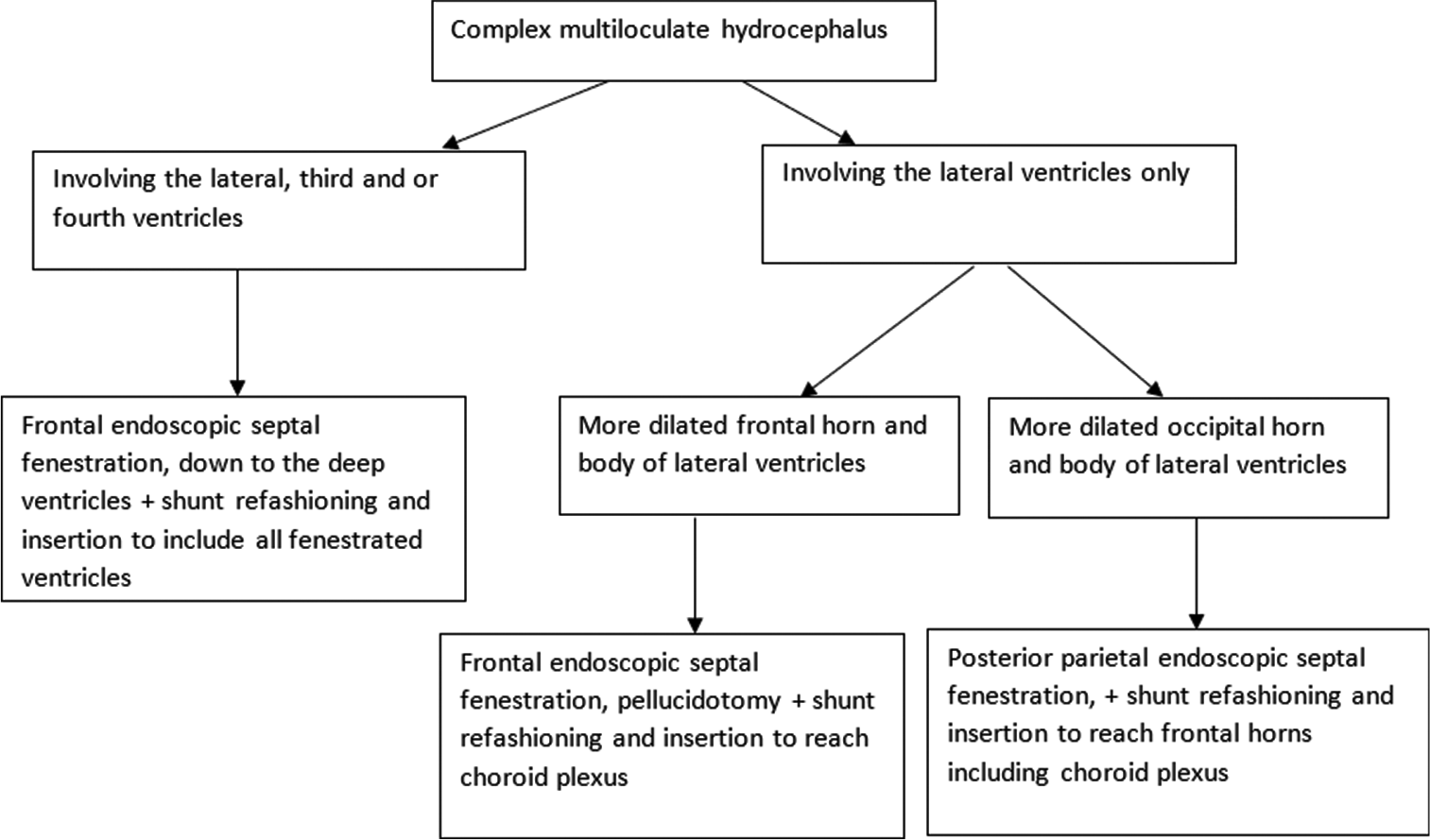
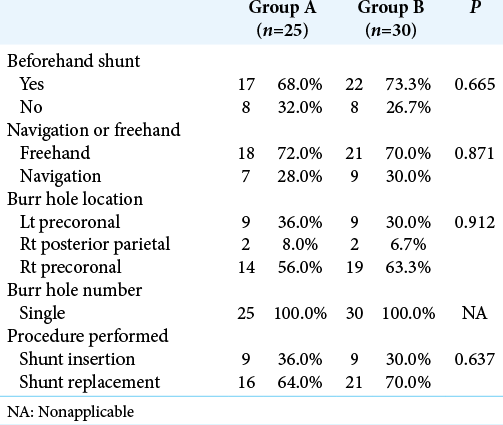
In the current study, patients were divided into two groups based on chronological order of the surgery. Group A was operated upon using the standard technique of endoscopic multiseptal wide fenestration and final ventriculoperitoneal shunt insertion without proximal shunt tube modification (factory default), while we used all modifications in Group B.
All patients were operated on using the little Lotta® or Gaab® endoscopic system (Karl Storz Company, Tuttlingen, Germany) under general anesthesia with local lidocaine infiltration. Choice of the point of endoscope entry was dictated by preoperative imaging and best path of endoscope shaft gaining access to most loculi. The length of the proximal catheter was calculated based on the patient’s preoperative image [
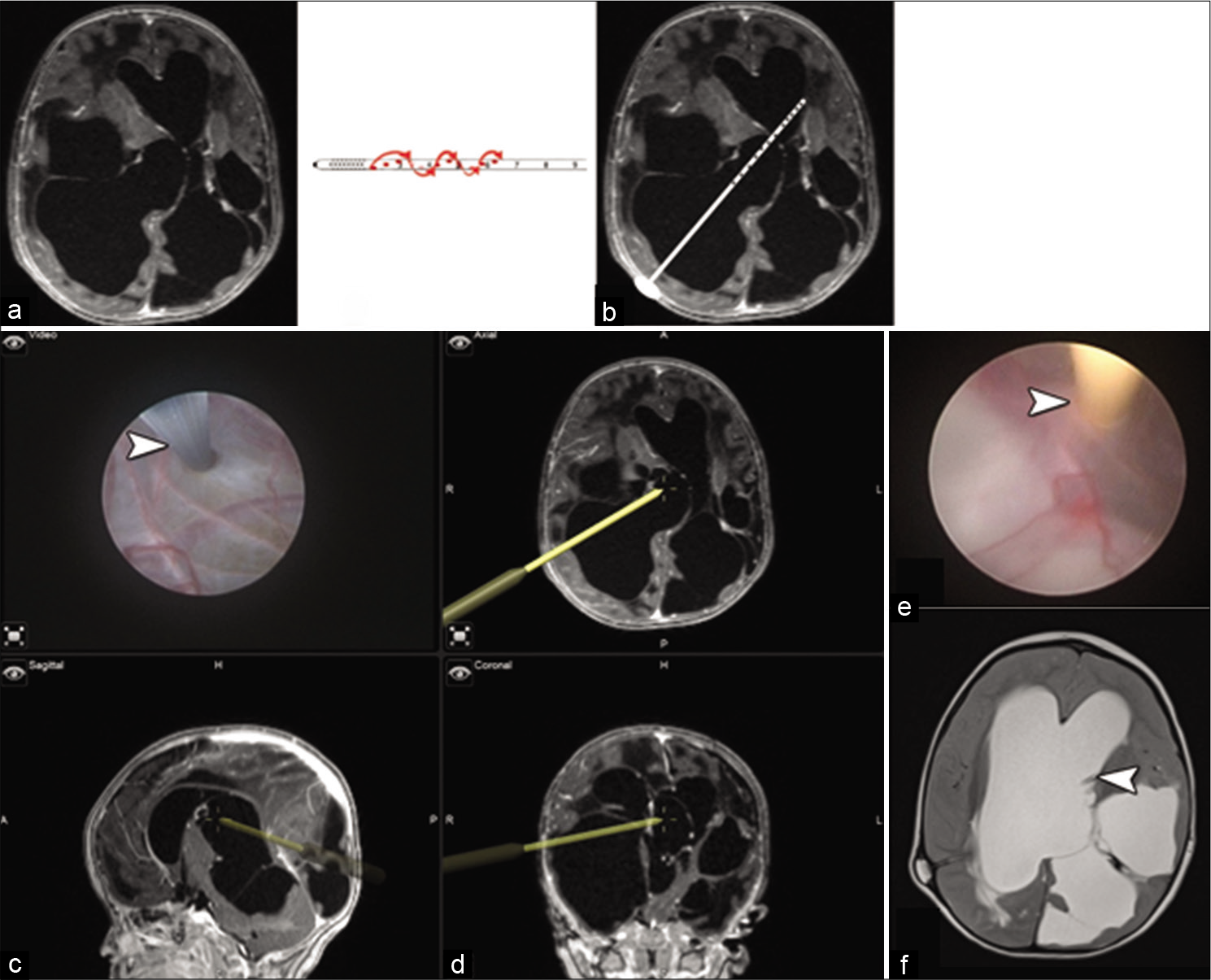
Figure 1:
(a) Axial T1-weighted MRI showing multiloculated hydrocephalus with asymmetric ventriculomegaly and a midline shift to the left side. (b) An illustration showing shunt tube refashioning by adding extra ports in all around manner to mimic the factory fashion. (c) An illustration showing the intended pathway and advancement of the proximal catheter into the ventricular system. (d) A screenshot from the navigation platform with four quadrants, the left upper corner is an endoscopic linked live closer view of the septum pellucidum being fenestrated by monopolar probe (white arrowhead). The rest of the quadrants are axial, sagittal, and coronal images showing the path of endoscope sheath used as navigation tool after registration on the system. (e) Insertion of the refashioned antibiotic impregnated proximal catheter (white arrowhead). (f) An axial T2-weighted MRI 3 months following surgery showing the proximal catheter tip on the contralateral ventricle as targeted (white arrowhead).
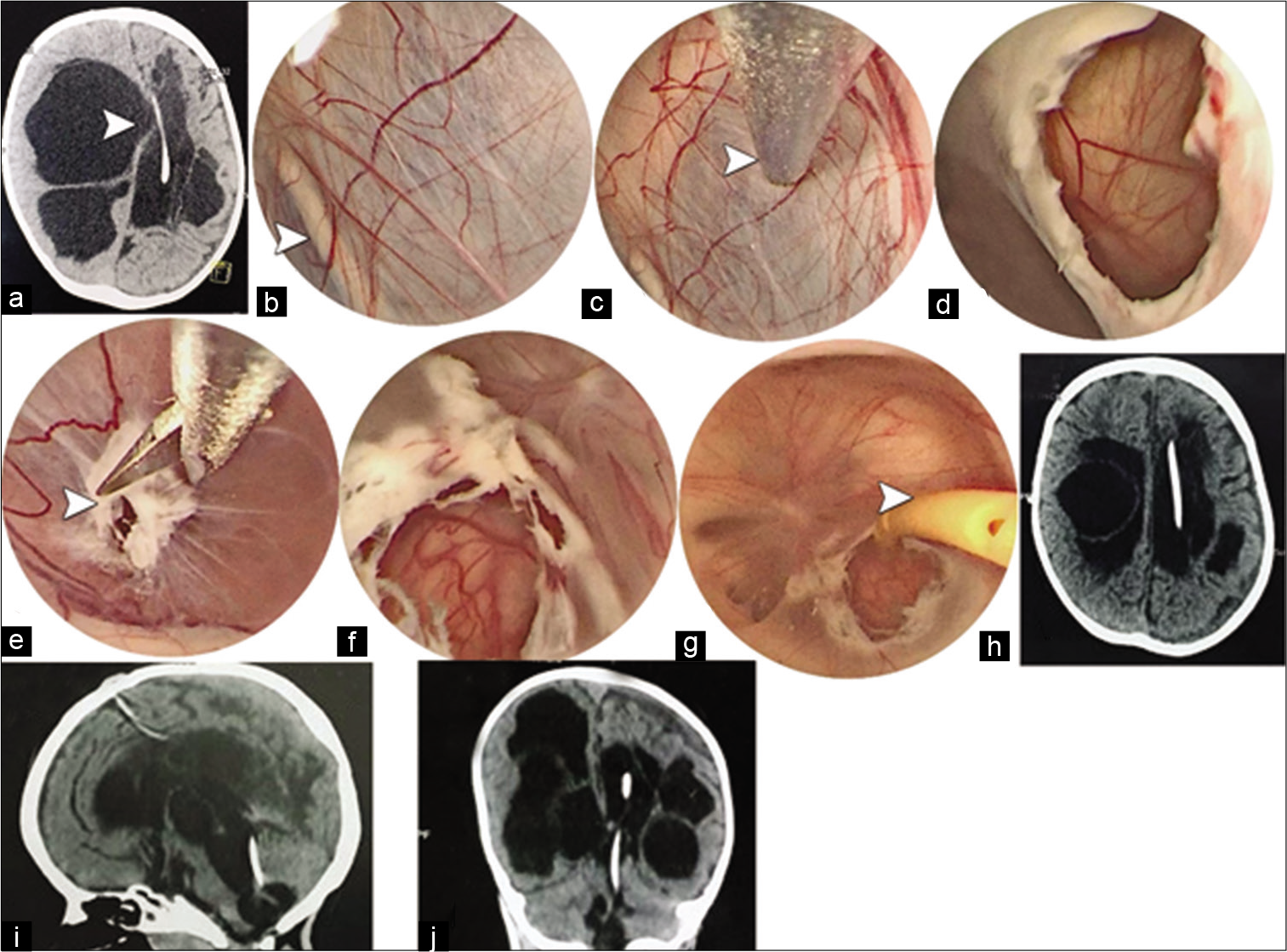
Figure 2:
(a) Axial CT brain showing multiloculated hydrocephalus with distention of the right lateral ventricle toward left side proximal shunt catheter (white arrowhead). (b) An intraoperative endoscopic right side view showing the left side proximal catheter behind the septum pellucidum (white arrowhead). (c) The wall of the septum pellucidum is fenestrated using bipolar probe. (d) The fenesetram is widely dilated to show the second leaflet of the septum pellucidum needs fenestration. (e and f) Fenestration and wide dilatation are further progressed down across ependymal adhesions isolating the dilated third and fourth ventricles (white arrowhead). (g) Insertion of the refashioned antibiotic impregnated proximal catheter throughout fourth, third, and lateral ventricles (white arrowhead). (h-j) Postoperative images showing the path of the catheter through the ventricular system with a resolution of midline shift.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (IBM SPSS Statistics) for Windows, version 26 (IBM Corp., Armonk, N.Y., USA). Age was abnormally distributed and summarized as median and interquartile range (IQR; expressed as 25th–75th percentiles) and compared between the two groups using Mann–Whitney U-test. Categorical variables were summarized as frequencies. Pearson’s Chi-square test for independence, Fisher’s exact test, or Fisher-Freeman-Halton exact test were used to examine association between groups and categorical variables. P < 0.05 was adopted to interpret results of statistical tests.
RESULTS
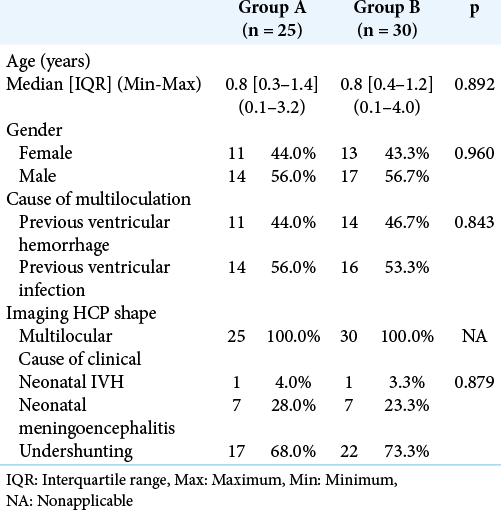
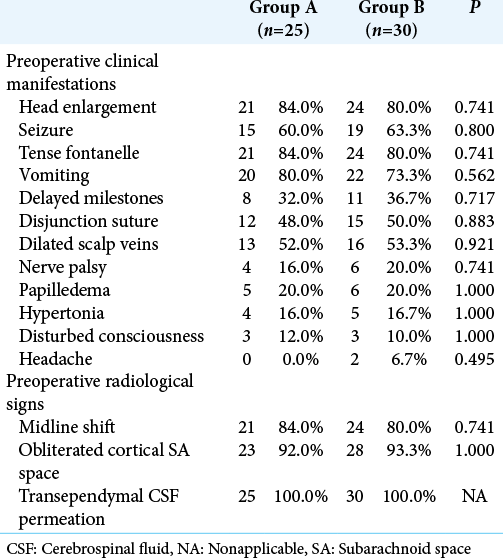
The present study was carried out on 55 patients who were enrolled into two groups: Group A (25 patients) and Group B (30 patients). [
[
[
[
[
Redo surgery was not significantly associated with the cause of MLH. In Group A, redo surgery was performed in 42.9% of postinfectious MLH compared to 45.5% of cases caused by hemorrhage (P = 1.000). In Group B, the rate of redo surgery was also higher in posthemorrhagic than in postinfectious cases (35.7% vs. 6.3%, P = 0.072). The presence of existing shunt was significantly associated with redo surgeries in Group A (P = 0.003), but nonsignificantly in Group B (P = 0.155) as all cases requiring redo surgery had preexisting shunt. The use of freehand versus intraoperative navigation was nonsignificantly associated with redo surgery in Groups A (50% vs. 28.6%, P = 0.407) and B (23.8% vs. 11.1%, P = 0.637).
DISCUSSION
Patients with MLH are at high risk of morbidity due to the recurrent nature of the pathological septations, which frequently necessitates the placement of multiple shunts and/ or redo surgeries or shunt revisions.[
Several treatment strategies have been assessed for managing MLH including multiple shunt insertion into each dilated loculation, fenestration of cysts by open surgery or endoscopy, or a combination of these techniques.[
The goal of the fenestration procedure is to make communication between the maximal possible number of isolated parts of the ventricular system creating openings in the septal walls so as CSF drainage will be achieved by the minimal number of shunts; this, in turn, will lessen the frequency of shunt revisions.[
The present study aimed at assessing the potential added benefit of a modified technique of endoscopic fenestration of cysts. The present study was carried out on 55 patients who either underwent the standard technique (Group A) or the modified technique (Group B). The two groups were similar as regards the preoperative characteristics and clinical manifestations. In Group B, patients underwent septal fenestration and pellucidotomy combined with proximal shunt tube refashioning by adding extra side ports all around and further advancement into isolated loculations of the ventricular system containing choroid plexus. Zohdi[
The etiology of MLH was previous ventricular infection in more than half the patients in either group, while the remaining cases were attributed to intraventricular hemorrhage. Ventriculitis, either due to infection or chemical irritation by intraventricular hemorrhage, leads to inflammation and damage of the ependyma. Consequently, gliosis develops with protrusion of glial tufts into the ventricular cavities that are composed of fibroglial tissue and inflammatory cells.[
Intraoperative navigation was used in the current study in only 28% and 30% of patients in Groups A and B, respectively. Intraoperative navigation has been recommended to improve localization of the cysts in complex MLH and to define the surgical approach and path to the targeted cysts.[
In both Groups A and B, the goal was wide fenestrations in the septal walls. First, the septa were cauterized, and then, the fenestrations were enlarged with Fogarty balloon and scissors. The aim of these steps was to reduce the likelihood of reclosure of the fenestrations which occurs frequently with small openings due to the low-pressure differential across loculated walls. The same precautions were recommended by El-Ghandour[
The use of endoscopic approach has been associated with reduced rate of perioperative morbidity due to the minimal invasiveness compared to open surgery through craniotomy. However, intraoperative bleeding which is less efficiently managed during endoscopy represents a concern that can affect the outcome of surgery.[
Spennato et al.[
El-Ghandour[
Eshra[
Kim et al.[
El-Tantawy[
Piyachon et al.[
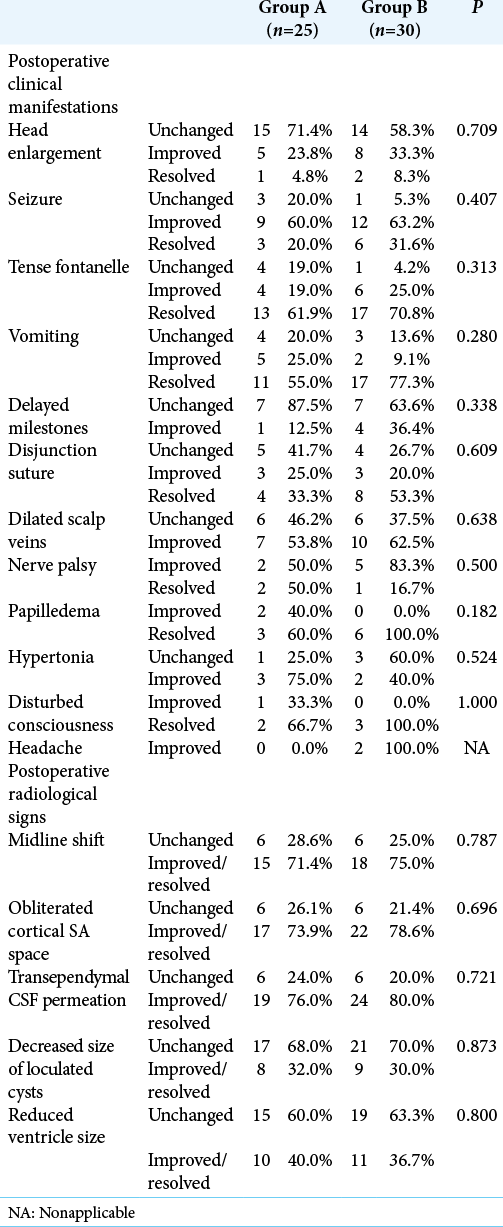
As regard the resolution of clinical and radiological manifestations after surgery, Group B showed a higher rate of improvement of almost all manifestations of increased intracranial pressure compared to Group A, but the difference was not statistically significant. The degree of improvement in achieving milestones was less evident, which is anticipated in these cases. Previous reports stated that the improvement in cognitive function and intelligence quotient did not parallel the rapid improvement in manifestations of increased intracranial pressure.[
Minimizing the number of inserted shunts can impact patients’ outcome. The presence of more than 1 shunt complicates the identification of a malfunctioning part. Moreover, the likelihood of shunt infection correlates with the number of shunts. Consequently, the insertion of a single shunt is preferable to avoid these complications.[
Another important outcome in cases of MLH is the need for redo surgeries or shunt revisions. The recurrent nature of the disease prevents the complete avoidance of these procedures. Redo surgery was performed in a lower percentage of patients in Group B (20% vs. 44%), but this difference did not reach statistical significance. The rate of redo surgeries widely varied among previous studies. A low rate of 16.7% (five patients) was reported by Fadl et al.,[
All patients in the current study who required redo surgery had existing shunts before endoscopic fenestration. This is conforming with Piyachon et al.[
CONCLUSION
The current study introduced a novel modified technique of endoscopic fenestration of cysts for the management of MLH. Although statistical significance was not detected between the two groups, the clinical significance of the reduced number of inserted shunts and redo surgery suggests an advantage of the modified technique while no added risk was detected. Consequently, we consider that the proposed modified technique has a promising potential of improving outcomes of MLH patients. Shunt hardware manufacturers can also participate in better management of these cases by providing dedicated factory longer fenestrated proximal catheters. The retrospective nature of the study might have impacted the results, and a randomized trial with special factory longer fenestrated proximal tube is recommended, taking into consideration the inclusion of adequate sample size to detect the differences between the tested techniques in terms of the number of inserted shunts and redo surgeries.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akbari SH, Holekamp TF, Murphy TM, Mercer D, Leonard JR, Smyth MD. Surgical management of complex multiloculated hydrocephalus in infants and children. Childs Nerv Syst. 2015. 31: 243-9
2. Albanese V, Tomasello F, Sampaolo S. Multiloculated hydrocephalus in infants. Neurosurgery. 1981. 8: 641-6
3. Andresen M, Juhler M. Multiloculated hydrocephalus: A review of current problems in classification and treatment. Childs Nerv Syst. 2012. 28: 357-62
4. Berman PH, Banker BQ. Neonatal meningitis. A clinical and pathological study of 29 cases. Pediatrics. 1966. 38: 6-24
5. Brown LW, Zimmerman RA, Bilaniuk LT. Polycystic brain disease complicating neonatal meningitis: Documentation of evolution by computed tomography. J Pediatr. 1979. 94: 757-9
6. Chrastina J, Novak Z, Riha I. Neuroendoscopy. Bratisl Lek Listy. 2008. 109: 198-201
7. Cipri S, Gambardella G. Neuroendoscopic approach to complex hydrocephalus. Personal experience and preliminary report. J Neurosurg Sci. 2001. 45: 92-6
8. El-Ghandour NM. Endoscopic cyst fenestration in the treatment of multiloculated hydrocephalus in children. J Neurosurg Pediatr. 2008. 1: 217-22
9. El-Tantawy MH. Endoscopic fenestration of multiloculated post-infectious hydrocephalus. Med J Cairo Univ. 2018. 86: 2995-3002
10. Eller TW, Pasternak JF. Isolated ventricles following intraventricular hemorrhage. J Neurosurg. 1985. 62: 357-62
11. Elsharkawy AA, Elatrozy H. Endoscopic antegrade aqueductoplasty and stenting with panventricular catheter in management of trapped fourth ventricle in patients with inadequately functioning supratentorial shunt. Surg Neurol Int. 2020. 11: 393
12. Eshra MA. Endoscopic management of septated, multiloculated hydrocephalus. Alex J Med. 2014. 50: 123-6
13. Fadl KN, Kasim AK, Abo Zeid W. Neuro-endoscopic management of multiloculated hydrocephalus. Sohag Med J. 2018. 22: 151-5
14. Harter DH. Management strategies for treatment of the trapped fourth ventricle. Childs Nerv Syst. 2004. 20: 710-6
15. Heilman CB, Cohen AR. Endoscopic ventricular fenestration using a “saline torch”. J Neurosurg. 1991. 74: 224-9
16. Jamjoom AB, Mohammed AA, Al-Boukai A, Jamjoom ZA, Rahman N, Jamjoom HT. Multiloculated hydrocephalus related to cerebrospinal fluid shunt infection. Acta Neurochir (Wien). 1996. 138: 714-9
17. Kim SA, Letyagin GV, Danilin VE, Sysoeva AA, Rzaev JA, Moisak GI. The benefits of navigated neuroendoscopy in children with multiloculated hydrocephalus. Asian J Neurosurg. 2017. 12: 483-8
18. Lee YH, Kwon YS, Yang KH. Multiloculated hydrocephalus: Open craniotomy or endoscopy?. J Korean Neurosurg Soc. 2017. 60: 301-5
19. Marquardt G, Setzer M, Lang J, Seifert V. Delayed hydrocephalus after resection of supratentorial malignant gliomas. Acta Neurochir (Wien). 2002. 144: 227-31
20. Mathiesen T, Grane P, Lindquist C, von Holst H. High recurrence rate following aspiration of colloid cysts in the third ventricle. J Neurosurg. 1993. 78: 748-52
21. Nida TY, Haines SJ. Multiloculated hydrocephalus: Craniotomy and fenestration of intraventricular septations. J Neurosurg. 1993. 78: 70-6
22. Nowosławska E, Polis L, Kaniewska D, Mikołajczyk W, Krawczyk J, Szymański W. Effectiveness of neuroendoscopic procedures in the treatment of complex compartmentalized hydrocephalus in children. Childs Nerv Syst. 2003. 19: 659-65
23. Oi S, Abbott R. Loculated ventricles and isolated compartments in hydrocephalus: Their pathophysiology and the efficacy of neuroendoscopic surgery. Neurosurg Clin N Am. 2004. 15: 77-87
24. Oi S, Hidaka M, Honda Y, Togo K, Shinoda M, Shimoda M. Neuroendoscopic surgery for specific forms of hydrocephalus. Childs Nerv Syst. 1999. 15: 56-68
25. Piyachon S, Wittayanakorn N, Kittisangvara L, Tadadontip P. Treatment of multi-loculated hydrocephalus using endoscopic cyst fenestration and endoscopic guided VP shunt insertion. Childs Nerv Syst. 2019. 35: 493-9
26. Ross DA, Muraszko K, Dauser R. A special cyst puncture catheter for use in thick-walled or mobile intracranial cysts. Neurosurgery. 1994. 34: 191-2
27. Sandberg DI, McComb JG, Krieger MD. Craniotomy for fenestration of multiloculated hydrocephalus in pediatric patients. Neurosurgery. 2005. 57: 100-6
28. Schulz M, Bohner G, Knaus H, Haberl H, Thomale UW. Navigated endoscopic surgery for multiloculated hydrocephalus in children. J Neurosurg Pediatr. 2010. 5: 434-42
29. Spennato P, Cinalli G, Ruggiero C, Aliberti F, Trischitta V, Cianciulli E. Neuroendoscopic treatment of multiloculated hydrocephalus in children. J Neurosurg. 2007. 106: 29-35
30. Teo C, Kadrian D, Hayhurst C. Endoscopic management of complex hydrocephalus. World Neurosurg. 2013. 79: S21.e1-7
31. Teo C, Rahman S, Boop FA, Cherny B. Complications of endoscopic neurosurgery. Childs Nerv Syst. 1996. 12: 248-53
32. Zohdi A.editors. Brain Endoscopy in Hydrocephalus the Role of Intracranial Endoscopy in the Management of Hydrocephalus; Dos and Dont’s (Doable or Not?). Germany: Tuttlingen Endo-Press; 2012. p.
33. Zuccaro G, Ramos JG. Multiloculated hydrocephalus. Childs Nerv Syst. 2011. 27: 1609-19