- Department of Neurosurgery, Ehime University School of Medicine, Toon, Japan.
- Department of Hematology, Clinical Immunology and Infectious Diseases, Ehime University School of Medicine, Toon, Japan.
- Division of Diagnostic Pathology, Ehime University School of Medicine, Toon, Japan.
- Department of Neurology and Geriatric Medicine, Ehime University School of Medicine, Toon, Japan.
Correspondence Address:
Akihiro Inoue, Department of Neurosurgery, Ehime University School of Medicine, Toon, Japan.
DOI:10.25259/SNI_8_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Seiji Shigekawa1, Akihiro Inoue1, Yukihiro Miyazaki2, Mashio Taniwaki3, Kota Kanehisa1, Sayaka Matsumoto4, Yoko Okada4, Riko Kitazawa3, Takeharu Kunieda1. Essentials for early diagnosis of primary intramedullary spinal cord lymphoma. How to suspect primary intramedullary spinal cord lymphoma early and proceed to invasive biopsy? A case report and literature review. 09-Feb-2024;15:41
How to cite this URL: Seiji Shigekawa1, Akihiro Inoue1, Yukihiro Miyazaki2, Mashio Taniwaki3, Kota Kanehisa1, Sayaka Matsumoto4, Yoko Okada4, Riko Kitazawa3, Takeharu Kunieda1. Essentials for early diagnosis of primary intramedullary spinal cord lymphoma. How to suspect primary intramedullary spinal cord lymphoma early and proceed to invasive biopsy? A case report and literature review. 09-Feb-2024;15:41. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12734
Abstract
Background: Primary intramedullary spinal cord lymphoma (PISCL) is an extremely rare condition. Early diagnosis is very difficult due to the nonspecific clinical and imaging findings. A biopsy is essential for a definitive diagnosis, but courage is required to perform the surgery. Here, we present a case of PISCL and suggest useful indicators for accurate diagnosis of this pathological entity.
Case Description: A 70-year-old woman presented with subacute bilateral lower-limb paralysis, disturbance of warm and pain sensations, and vesicorectal disturbance. Magnetic resonance imaging showed a contrast-enhanced mass from C7 to Th2 and large, edematous lesions from the upper cervical to lower thoracic spinal cord. Elevated uptake of 18F-fluoro-2-deoxy-D-glucose (FDG) was identified in the enhanced regions on FDG-positron emission tomography (PET). Cerebrospinal fluid (CSF) analysis revealed highly elevated levels of β2-microglobulin (β2-MG). Steroid pulse therapy and therapeutic plasma exchange were performed for suspected myelitis, but symptoms did not improve. Spinal cord biopsy was, therefore, performed for treatment-resistant myelopathy. Histopathological examination revealed diffuse large B-cell lymphoma, which was diagnosed as PISCL because systemic examination showed no other findings suggestive of malignant lymphoma.
Conclusion: In cases with poor response to treatment and a progressive course, PISCL should be considered, and spinal cord biopsy should be performed if PET shows increased 18F-FDG uptake and β2-MG is elevated in CSF.
Keywords: Accurate diagnosis, Positron emission tomography, Primary intramedullary spinal cord lymphoma, Surgical biopsy, β2-microglobulin
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is rare, accounting for <1% of all lymphomas. It is confined to the eye, brain, leptomeninges, and spinal cord, with no systemic involvement.[
CASE DESCRIPTION
General remarks
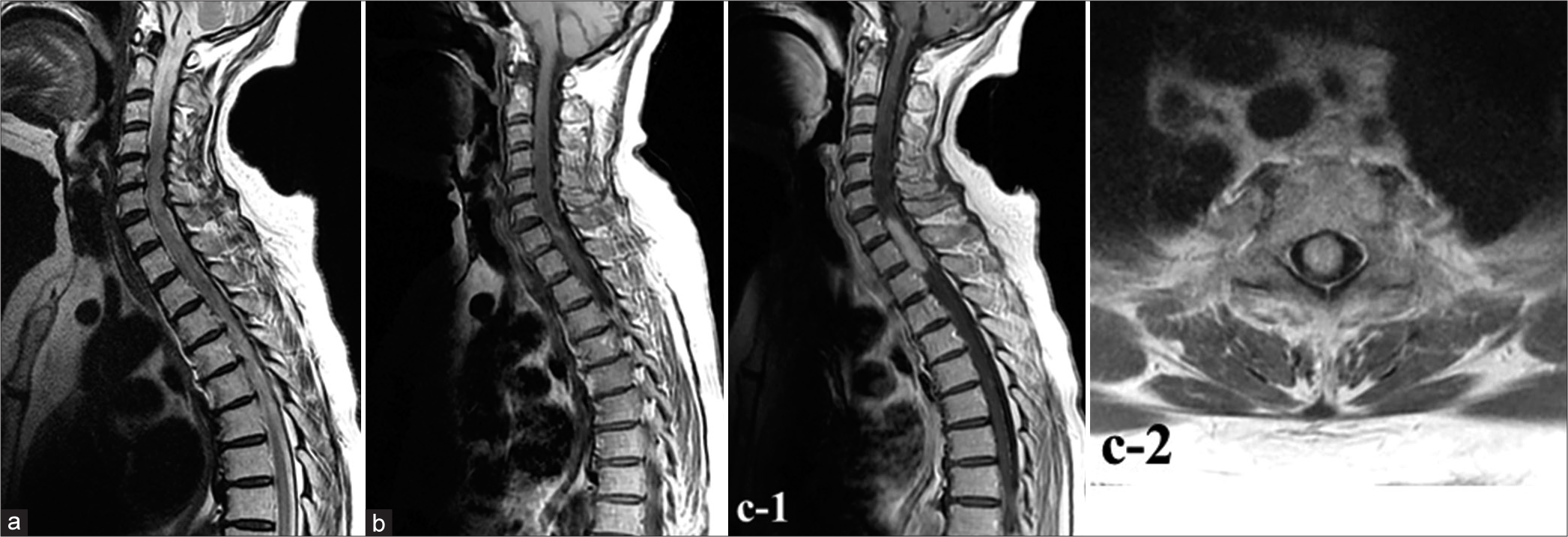
A 70-year-old woman presented with a 4-month history of numbness in the left chest that had gradually worsened and then spread to both lower extremities with associated gait disturbance. She was, therefore, admitted to our hospital. She had no obvious history of trauma, systemic diseases, or metabolic disorders. Neurological examination on admission revealed dysesthesia, decreased warm and pain sensations in the thoracic (Th)4 vertebral region and lower and hyperreflexia in both lower limbs. Complete paralysis and pronounced numbness were apparent in both lower limbs. Magnetic resonance imaging (MRI) showed a longitudinally spreading, intradural spinal cord lesion in the cervical (C)7–Th2 region, with gadolinium (Gd) enhancement on T1-weighted imaging (WI) with diffuse swelling within the C2–Th6 levels [
Figure 1:
Results of magnetic resonance imaging (MRI) on admission. MRI shows an intradural, longitudinally spreading spinal cord lesion in the cervical (C)7 to thoracic (Th)2 region, with gadolinium enhancement on T1-weighted imaging (WI) and diffuse swelling from the C2 to Th6 level. (a) Sagittal T2-weighted imaging. (b) Sagittal T1-weighted imaging (T1-WI) with non-contrast sequence. (c-1) Sagittal gadolinium-enhanced T1-WI. (c-2) Axial Gd-enhanced T1-WI.
Progress of treatment
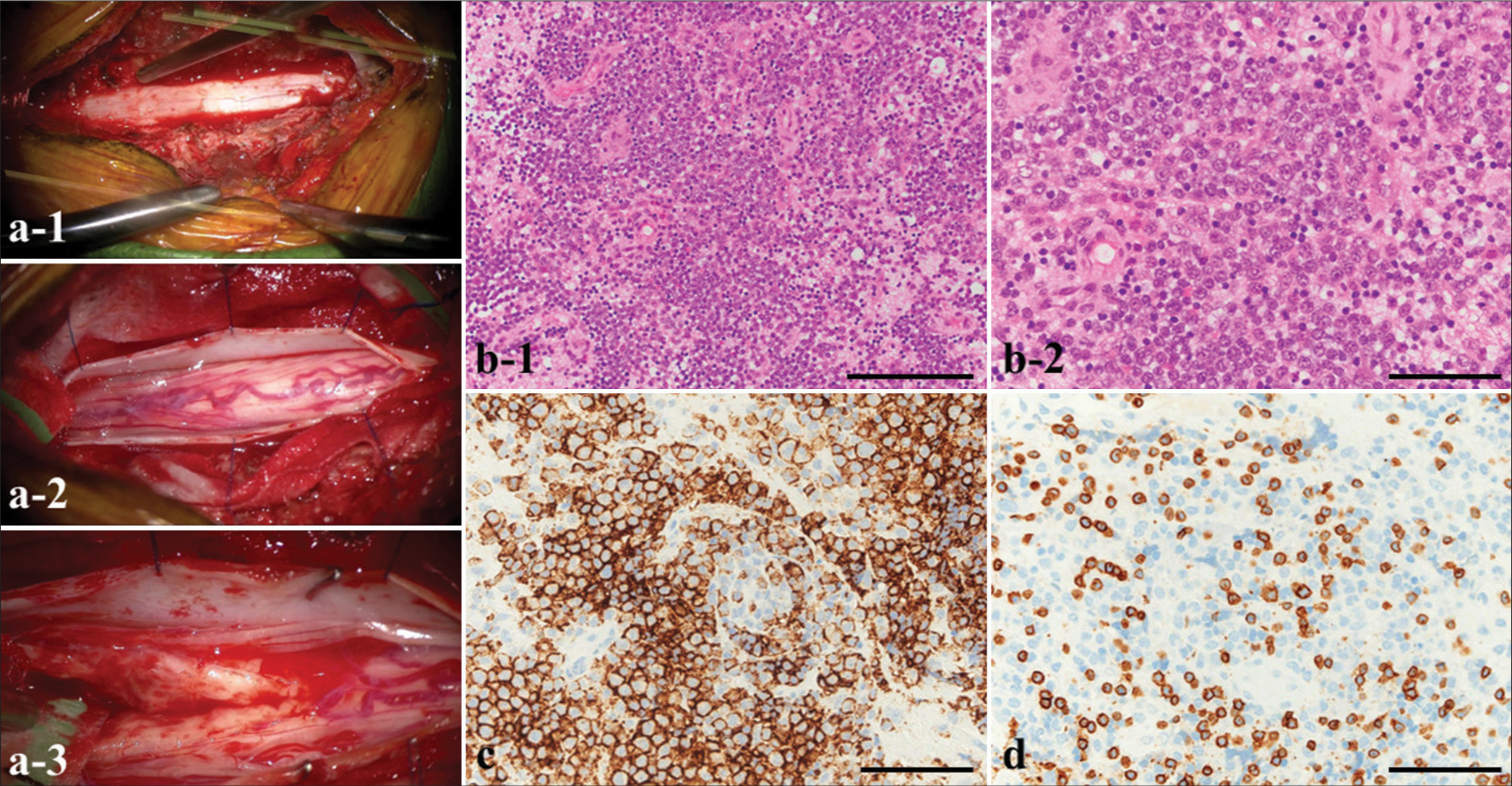
Transverse myelitis was initially suspected, and steroid pulse therapy and simple plasma exchange therapy were performed, but no symptom improvement was obtained. Therefore, to confirm the histological diagnosis and plan effective treatment for the primary disease, a surgical biopsy of the lesion was performed under a posterior approach (total Th1–Th3 laminectomy). The dura mater was incised to identify the spinal cord; then, gentle blunt dissection of the posterior median sulcus was performed to locate the lesion. The lesion did not show an obvious macroscopic margin, but abnormal tissue was easily removed for biopsy [
Figure 3:
(a) Intraoperative photographs. (a-1) Total laminectomy (Th1–3). (a-2) The dura mater is incised to identify the spinal cord. (a-3) Gentle, blunt dissection of the posterior median sulcus is performed to locate the lesion, which does not show an obvious macroscopic margin. (b ) Histopathology of the resected tumor (b-1 and b-2) shows that large, atypical lymphocytes have extensively infiltrated the lesions. (c) The cells reveal positive staining for a cluster of differentiation (CD) 20 (d) and negative staining for CD3. Magnifications: (b-1) ×100; (b-2, c, d) ×400. Scale bars: (b-1) 400 µm; (b-2, c, d) 100 µm.
Postoperative course
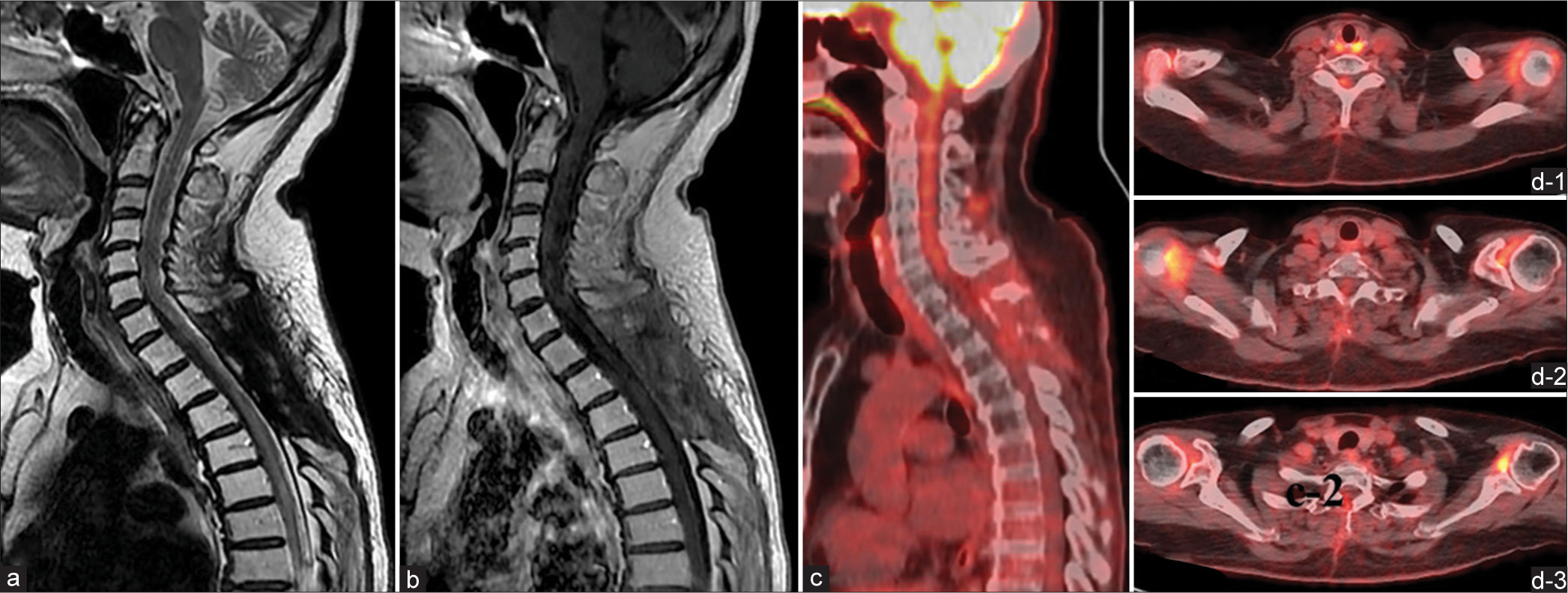
The patient was placed on rituximab, methotrexate, procarbazine, and vincristine (R-MPV regimen). He was administered a total of four cycles, but serial MRI during the care process did not show any tumor shrinkage effect, so local radiation therapy was performed (40 Gy in 20 fractions). The subsequent course was uneventful, with MRI and 18F-FDGPET at ten months after completion of chemoradiotherapy, showing a complete disappearance of Gd and 18F-FDG accumulation in the spinal cord [
DISCUSSION
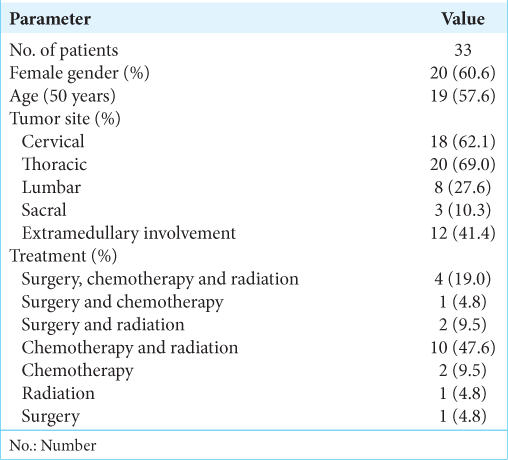
Malignant lymphoma arising in the CNS, as so-called PCNSL, is a rare form of extranodal non-Hodgkin lymphoma. The clinical characteristics of PISCL patients obtained from the literature review are shown in
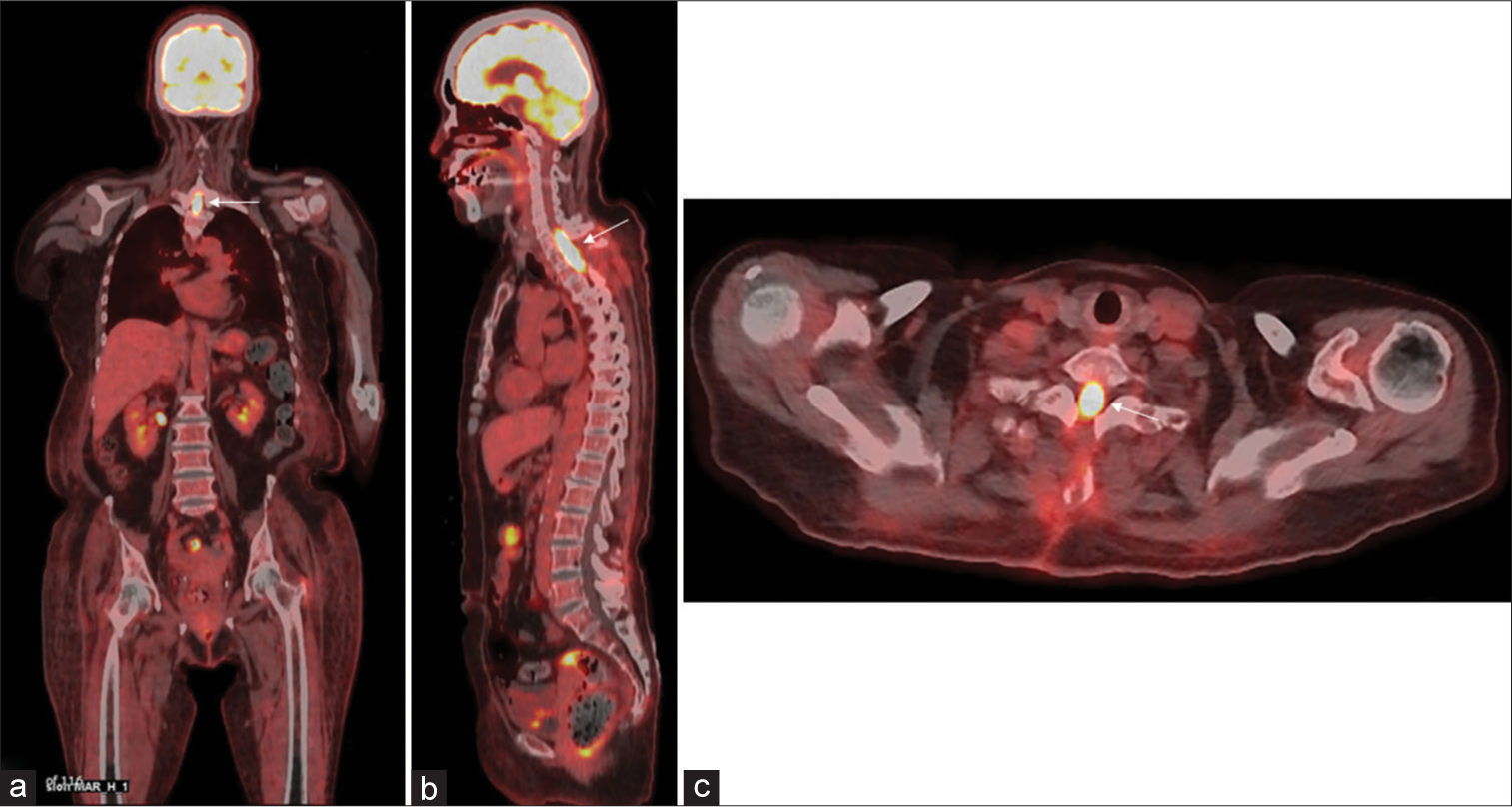
To date, no consensus has been reached on appropriate presurgical diagnostic evaluations. We consider that key points in the diagnosis of PISCL are imaging findings from 18F-FDG-PET and CSF.[
Regarding CSF findings, past reports have stated that C-X-C motif chemokine ligand 13 (CXCL13), interleukin (IL)-10, soluble IL-2 receptor (sIL-2R), and β2-MG in CSF show excellent diagnostic ability as biomarkers for PCNSL.[
Finally, few reports have dealt with the treatment of PISCL, and, at this point, no definitive methods have been identified. Surgery, radiation therapy, and chemotherapy are commonly used for PISCL, alone or in combination.[
CONCLUSION
We have reported an extremely rare case of PISCL, diagnosed and confirmed by early surgical biopsy and treated with chemoradiotherapy using the R-MPV regimen. In cases showing progressive spinal cord lesions of unknown cause, PISCL should be considered as a differential diagnosis. In particular, if MRI shows a uniform contrast effect and extensive edema is seen on T2-WI/MRI, β2-MG levels in CSF should be measured, and 18F-FDG-PET and early surgical biopsy performed to investigate the possibility of PISCL. The successful clinical management in this case provides an important potential option for treating PISCL patients.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Flanagan EP, O’Neill BP, Porter AB, Lanzino G, Haberman TM, Keegan BM. Primary intramedullary spinal cord lymphoma. Neurology. 2011. 77: 784-91
2. Garcia DM. Primary spinal cord tumors treated with surgery and postoperative irradiation. Int J Radiat Oncol Biol Phys. 1985. 11: 1933-9
3. Ge H, Xu L, Gao H, Ji S. Primary intramedullary spinal cord lymphoma misdiagnosed as longitudinally extensive transverse myelitis: A case report and literature review. BMC Neurol. 2023. 23: 352
4. Hachicha A, Belhaj A, Karmeni N, Slimane A, Bouali S, Kallel J. Intramedullary spinal cord tumors: A retrospective multicentric study. J Craniovertebr Junction Spine. 2021. 12: 269-78
5. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004. 103: 275-82
6. Inoue A, Matsumoto S, Ohnishi T, Miyazaki Y, Kinnami S, Kanno K. What is the best preoperative quantitative indicator to differentiate primary central nervous system lymphoma from glioblastoma?. World Neurosurg. 2023. 172: e517-23
7. Kong L, Raunio S. Primary central nervous system lymphoma presenting as an isolated intramedullary spinal cord lesion: A case report. AME Case Rep. 2022. 7: 10
8. Lai R, Abrey LE, Rosenblum MK, DeAngelis LM. Treatment-induced leukoencephalopathy in primary CNS lymphoma: A clinical and autopsy study. Neurology. 2004. 62: 451-6
9. Maeyama M, Sasayama T, Tanaka K, Nakamizo S, Tanaka H, Nishihara M. Multi-marker algorithms based on CXCL13, IL-10, sIL-2 receptor, and β2-microglobulin in cerebrospinal fluid to diagnose CNS lymphoma. Cancer Med. 2020. 9: 4114-25
10. Okano A, Kanai M, Kita T, Nakai Y, Okada H, Yamaguchi K. A case of primary intramedullary spinal cord lymphoma diagnosed by spinal cord biopsy of long spinal cord lesions showing persistent gadolinium contrast enhancement. Rinsho Shinkeigaku (Clin Neurol). 2021. 61: 856-61
11. Scott BJ, Douglas VC, Tihan T, Rubenstein JL, Josephson SA. A systematic approach to the diagnosis of suspected central nervous system lymphoma. JAMA Neurol. 2013. 70: 311-9
12. Sivri M, Erdoğan H, Allahverdiyev I, Koplay M, Temizöz O. A rare cause of spinal mass: Primary intramedullary spinal cord lymphoma. Spine J. 2015. 15: e43-4
13. Wu Q, Yang Z, Xu Y. Nomograms predict survival outcome of primary intramedullary spinal cord lymphoma patients. Med Sci Monit. 2019. 25: 7418-29
14. Zhang G, Li J, Hui X. Use of 18F-FDG-PET/CT in differential diagnosis of primary central nervous system lymphoma and high-grade gliomas: A meta-analysis. Front Neurol. 2022. 13: 935459