- Department of Anaesthesia and Intensive Care, St. John’s Medical College, Bengaluru, Karnataka, India
- Department of Anesthesia and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Neurosurgery, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Rajeev Chauhan, Department of Anaesthesia and Intensive Care, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_874_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rajesh Kanan1, Rashi Sarna2, Neerja Bharti2, Nidhi Bidyut Panda2, Rajeev Chauhan2, Nidhi Singh2, Ankur Luthra2, Madhivanan Karthigeyan3. Evaluation of the changes in middle cerebral artery flow velocity related to different positions of patients during posterior fossa surgery. 18-Nov-2022;13:541
How to cite this URL: Rajesh Kanan1, Rashi Sarna2, Neerja Bharti2, Nidhi Bidyut Panda2, Rajeev Chauhan2, Nidhi Singh2, Ankur Luthra2, Madhivanan Karthigeyan3. Evaluation of the changes in middle cerebral artery flow velocity related to different positions of patients during posterior fossa surgery. 18-Nov-2022;13:541. Available from: https://surgicalneurologyint.com/surgicalint-articles/12005/
Abstract
Background: This is a prospective observational study to evaluate the changes in middle cerebral artery flow velocities and cerebral perfusion pressure in the various positions used for posterior cranial fossa surgery and to correlate these changes with postoperative recovery characteristics and complications.
Methods: Sixty patients were included in the study – 33 patients with CPA tumors were placed in the supine with head tilt position and the rest 27 with tumors in other locations of posterior fossa were placed in the prone position. The primary aim was to study the changes in middle cerebral artery blood flow velocity related to various positions of the patients used during posterior fossa surgery. The secondary aim was to compare the changes in pulsatility index, resistance index, and effective cerebral perfusion pressure in different position and to correlate these findings with postoperative recovery and the complications associated with these positions.
Results: The systolic and mean flow velocities were higher in the supine with head tilt group than the prone group after positioning and post repositioning, but these values were within normal limits, and the changes with positioning from baseline were comparable between the groups. Furthermore, these changes did not affect the effective cerebral perfusion pressure or the outcomes of the patients.
Conclusion: The current results do not determine whether the supine with head tilt position is better than the prone position during posterior fossa surgery.
Keywords: American society of anesthesiologists, Cerebellopontine angle, Posterior fossa surgery, Prone position, Supine with head tilt position
INTRODUCTION
The posterior fossa can be done in various positions including sitting position, supine with lateral head tilt, prone, lateral, three quarter prone, and park-bench position.[
MATERIALS AND METHODS
After approval from the Institutional Ethics Committee (NK/3831/MD/439 dated October 28, 2017) and prospective registration with the Clinical Trials Registry of India (CTRI/2018/01/011206), this prospective and observational study was conducted in 60 ASA I-III (ASA I- A normal healthy patient, ASA II- A patient with mild systemic disease, and ASA III- A patient with severe systemic disease) patients aged between 18 and 65 years undergoing posterior fossa surgery for various pathologies between July 2017 and December 2018 at a tertiary care center in North India. Patients with preoperative GCS < 13, history of carotid artery disease, ischemic stroke, unstable cardiorespiratory disorders, and renal or hepatic insufficiency were excluded from the study. After taking the patient inside the operation theatre, standard ASA monitors were attached. A baseline heart rate (HR), oxygen saturation, and blood pressure were noted. Intravenous access was taken and anesthesia was induced with propofol (1.5–2.5 mg/kg) and morphine (0.1 mg/kg). Neuromuscular paralysis was achieved with vecuronium (0.1 mg/kg) and an appropriately sized endotracheal tube was placed. Anesthesia was maintained with isoflurane with O2 and N2O. Ventilator settings were adjusted to maintain end tidal carbon-di-oxide (EtCO2) between 30 and 35 mm Hg. All the patients received intravenous acetaminophen 15 mg/ kg over 10 min at the end of surgery for postoperative analgesia. After skin closure, isoflurane was discontinued. The operating surgeon was requested to choose the quality of the surgical field during the procedure from Boezaart’s scale [Annexure 1] of 1–5. After making the patient supine, the neuromuscular paralysis was reversed with neostigmine and glycopyrrolate and the patient was extubated if obeying verbal commands, taking regular, and spontaneous breaths of adequate tidal volume and was hemodynamically stable. Else the patient was mechanically ventilated. Postoperatively, patients were shifted to post anesthesia care unit if extubated and if not extubated to the neurosurgical ward for mechanical ventilation and followed up till discharge.
TCD with combined color flow and power Doppler was done at the following time points, through the trans-temporal window – (a) preoperatively before induction with the patient supine, (b) postinduction before positioning the patient, (c) after positioning of the patient in the desired surgical position, and (d) after the patient was made supine (before reversal).
After applying acoustic gel, TCD probe was applied at the temporal area of the patient. A signal was searched for, initially starting at a depth of 50 mm for a FV pulse waveform above and below the zero-line, characteristic of ICA with flow both toward and away from the probe. Signal gain was enhanced by slight adjustments of the insonation angle or by changing the depth (45–70 mm). The depth of insonation was then reduced back to 45–55 mm to identify waveform with only the positive deflection, characteristic of M1 part of the MCA FV waveform. The point of maximum amplitude of deflection was taken for measurements. Procedure was repeated on the other side.
The following parameters were recorded:
Systolic flow velocity (SFV), diastolic flow velocity (DFV), and mean flow velocity (MFV) of the middle cerebral arteries of both sides Pulsatility and resistance indices for these arteries Transient hyperemic response test (THRT) preoperatively.
Effective CPP (eCPP) was calculated using the formula
eCPP = MAP * (DFV/MFV) + 14 (mmHg).[
[MAP-Mean arterial pressure; DFV-Diastolic flow velocity, MFV-Mean flow velocity]
THRT was performed keeping the TCD probe in place recording the MCA flow velocity, while manually compressing the ipsilateral common carotid artery, with compression ratio of more than 40% for 10 s. Then, peak MCA flow velocity immediately after releasing the carotid compression was recorded. The ratio of the peak hyperemic flow velocity recorded after carotid release and the precompression baseline flow velocity was calculated. THRR > 1.10 was considered as intact autoregulation, while values < 1.10 were taken as deranged autoregulation. Three readings of THRR were taken at an interval of 2 min between the readings and the average of the 3 readings was taken as the final one. THRR was calculated on both the sides and noted.
Follow-up was done until 1-month postsurgery. Duration of ICU and hospital stay, duration of mechanical ventilation, extended Glasgow outcome score (GOS-E) at discharge and at 1 month, and any postoperative complications (hemorrhage, re-exploration, CSF leak, and meningitis) were recorded.
Statistical analysis
The statistical analysis was carried out using IBM Statistical Package for the Social Sciences statistical version 25. Sample size was calculated using the Minitab software. From the study by Hanouz et al.,[
RESULTS
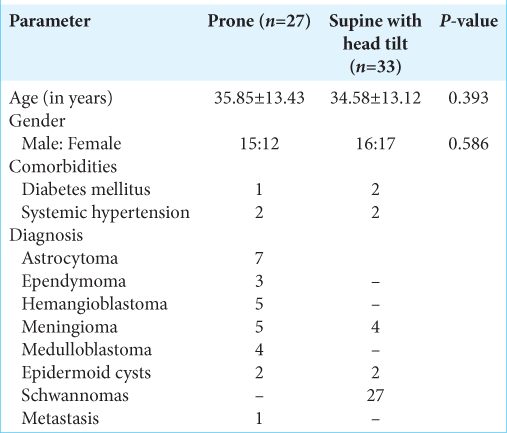
Of the total 60 patients enrolled in the study, 33 patients who had cerebellopontine angle tumors were placed in the supine position with head tilt to the side opposite of the side of tumor, whereas the other 27 patients were placed in the prone position. The distribution of demographic characters was similar in both the groups [
The hemodynamic parameters were maintained within the normal limits during the surgery. Although the HR deviated from the baseline on many occasions, there were only two episodes of bradycardia due to brainstem handling in both groups that needed intervention and these numbers were comparable. The mean arterial pressure also deviated from the baseline at many timepoints of observation. There was a significant fall postinduction in both groups. The HR and MAP readings were comparable between both the groups at all points. EtCO2 concentrations were within normal limits, though there was a decrease postpositioning and it was close to baseline values at the end of the surgery. The two groups did not differ significantly between themselves in terms of the EtCO2 concentrations.
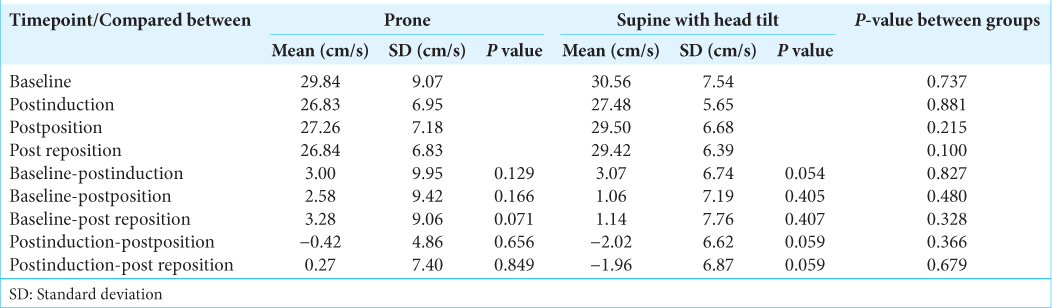
All patients had normal transient hyperemic response ratios in both the groups and had intact autoregulatory mechanisms that would maintain their cerebral blood flow despite changes in MAP. The values were also comparable on both the sides indicating no side to side discrepancy. The flow velocity parameters were within normal limits and when compared between the right and left sides within each group, they were comparable at all-time points of observation. Hence, there was no side to side discrepancy due to anesthesia and positioning. Pulsatility index and resistivity index measurements revealed normal values on both sides indicating a normal cerebrovascular resistance on both sides in both groups.
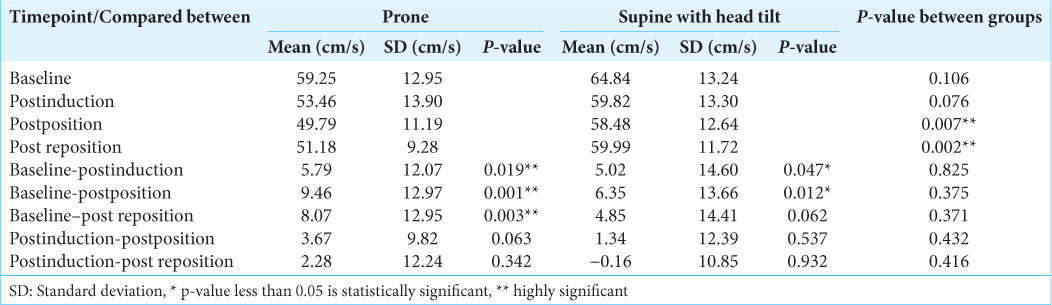
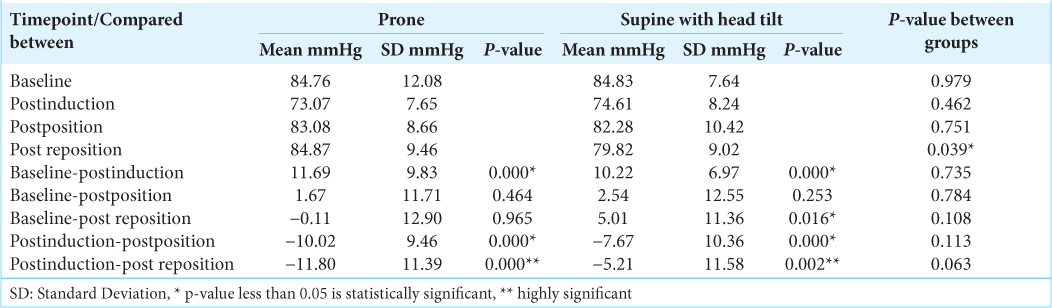
The SFV values and the mean flow velocities were significantly higher in supine with head tilt group than the prone group, after positioning and post repositioning. The diastolic flow velocities were comparable at all-time points. The SFVs as compared to baseline fell significantly postinduction and postpositioning in both the groups (P < 0.05) [
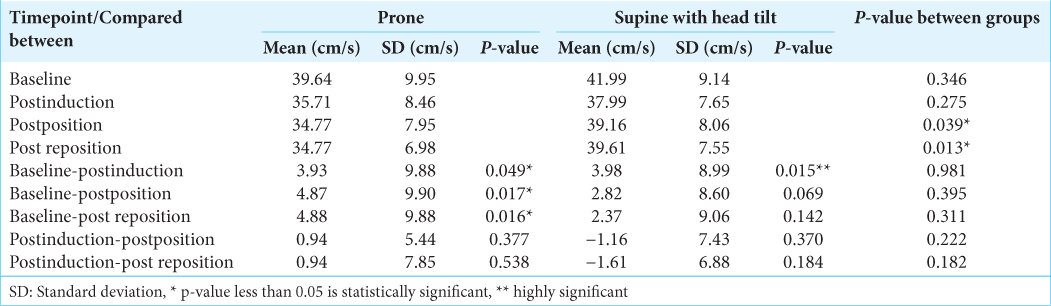
In both groups, the effective CPP was normal and was comparable at baseline, postinduction, and postpositioning [
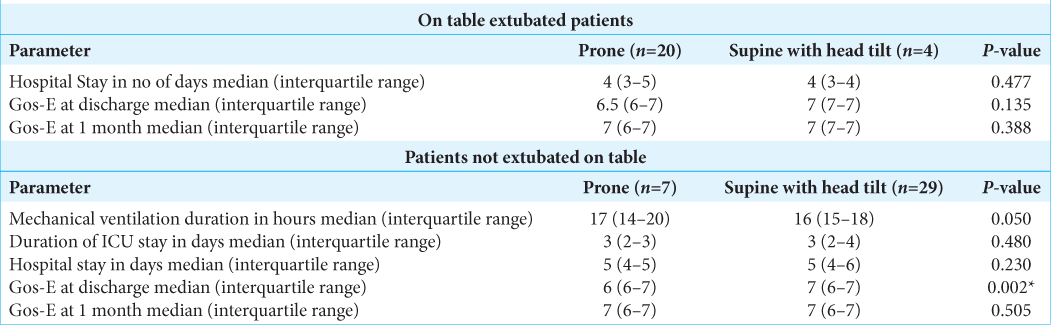
Both the groups, in our study, had comparable blood losses and satisfactory surgical field gradings. The duration of surgery and also the intravenous fluid requirement was higher in the supine with head tilt group as compared to prone group. A significantly greater proportion of the posterior fossa patients with lesser duration of surgery were extubated on table as compared to the supine with head tilt group who were mechanically ventilated electively due to greater surgical manipulation or presence of preoperative neurological deficits. Patients of supine with head tilt group had similar hospital stay and GOS-E score at discharge and at 1 month as compared to the prone group. In patients not extubated on table, two patients of the supine with head tilt group were tracheostomized. Otherwise, both groups had similar durations of ICU stay and hospital stay [
At discharge, the patients in the prone group had a lower GOS-E due to persistence of ataxia than the supine with head tilt group. However, at 1 month, they had similar GOS-E scores as CPA tumor patients as ataxia improved. No positioning related complications were recorded in this study.
DISCUSSION
The position during surgeries of intracranial tumors of the posterior fossa is mainly dictated by the location of the tumor and the position providing best access to the surgeon. Each of these positions is inherent with specific complications and can be due to the alterations in cerebral perfusion pressure in the various positions. Our aim, in this study, was to measure and compare the changes in the middle cerebral artery flow velocities that occur with positioning, among the various positions, in which the patients are placed for surgery of the posterior fossa. Our patients were placed in the supine with head tilt position for cerebellopontine angle tumors and in the prone position for other posterior fossa tumors.
In our study, the significant fall in systolic and mean flow velocities in both the groups postinduction paralleled the fall in mean arterial pressure in both the groups during induction. While there was a fall in systolic and mean flow velocities in the prone group postpositioning, in the supine with head tilt group, there was a significant fall in the SFV alone which did not reflect in a significant fall in the MFV. This could be due to the lesser changes in diastolic flow velocities in the supine with head tilt group than in the prone group. From the postinduction values, neither the SFV nor the MFV changed significantly after positioning and after repositioning in both the groups and the changes themselves were comparable between the groups. Hence, the changes in the systolic and MFV were due to the changes in mean arterial pressure and not due to positioning. The difference between the groups can be explained by the greater fall in mean arterial pressure in the prone group than the supine with head tilt group. Hanouz et al.,[
In a study in neurocritical care unit caring for postoperative patients by Kose and Hatipoglu,[
Bombardieri et al.[
The effective cerebral perfusion pressure was within normal limits in both the groups at all-time points. The effective cerebral perfusion pressure was lower in the supine with head tilt group than the prone group after repositioning. This change could be due to surgical manipulation. The effective cerebral perfusion pressure decreased in both the groups postinduction and returned close to baseline after positioning and after repositioning, demonstrating an increase. These changes paralleled the changes in the mean arterial pressure. All changes in eCPP in both the groups whether from baseline or from postinduction values to the later time points were comparable.
However, some of the limitations of our study were (a) we could not measure the flow velocities continuously during intraoperative period which requires a fixation device (helmet), as it would have interfered with the surgical positioning and pin application. (b) No patients were subjected to surgery in the sitting, beach chair or lateral positions; hence, the effect of positioning could not be compared with respect to these positions. (c) Our study was a single center study. A multicenter study with a larger sample size would better demonstrate changes with different surgical positions.
CONCLUSION
We found that the systolic and mean flow velocities were higher in the supine with head tilt group than the prone group after positioning and post repositioning, but these values were within normal limits, and the changes with positioning from baseline were comparable between the groups. Furthermore, these changes did not affect the effective cerebral perfusion pressure or the outcomes of the patients. Therefore, the current results do not allow us to determine whether the supine with head tilt position is better than the prone position.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SURGICAL FIELD GRADING (BOEZAART’S SCALE)
0 No bleeding.
1 Slight bleeding - no suctioning of blood required.
2 Slight bleeding- occasional suctioning required. Surgical field not threatened.
3 Slight bleeding- frequent suctioning required. Bleeding threatens surgical field a few seconds after suction is removed
4 Moderate bleeding- frequent suctioning required. Bleeding threatens surgical field directly after suction is removed
5 Severe bleeding- constant suctioning required. Bleeding appears faster than can be removed by suction. Surgical field severely threatened and surgery not possible
A Boezaart’s score of less than 4 will be considered as surgeon’s satisfaction.
References
1. Black S, Ockert DB, Oliver WC, Cucchiara RF. Outcome following posterior fossa craniectomy in patients in the sitting or horizontal positions. Anesthesiology. 1988. 69: 49-56
2. Bombardieri AM, Beckman J, Urban M, Go G, De Gaudio AR, Girardi FP. An observational study of cerebral blood flow velocity evaluation in the prone position during posterior lumbar surgery. Anesth Analg. 2019. 129: 487-92
3. Calliauw L, Van AJ, Rolly G, Verbeke L. The position of the patient during neurosurgical procedures on the posterior fossa. Acta Neurochir. 1987. 85: 154-8
4. Cardim D, Robba M, Bohdanowicz M, Donnelly J, Cabella B, Liu X. Non-invasive monitoring of intracranial pressure using transcranial doppler ultrasonography: Is it possible?. Neurocrit Care. 2016. 25: 473-91
5. D’Andrea A, Conte M, Cavallaro M, Scarafile R, Riegler L, Cocchia R. Transcranial Doppler ultrasonography: From methodology to major clinical applications. World J Cardiol. 2016. 8: 383-400
6. Hanouz JL, Fiant AL, Gérard JL. Middle cerebral artery blood flow velocity during beach chair position for shoulder surgery under general anesthesia. J Clin Anesth. 2016. 33: 31-6
7. Kose G, Hatipoglu S. Effect of head and body positioning on cerebral blood flow velocity in patients who underwent cranial surgery. J Clin Nurs. 2012. 21: 1859-67
8. Purkayastha S, Sorond F. Transcranial Doppler ultrasound: Technique and application. Semin Neurol. 2012. 32: 411-20
9. Rath GP, Bithal PK, Chaturvedi A, Dash HH. Complications related to positioning in posterior fossa craniectomy. J Clin Neurosci. 2007. 1: 520-5
10. Smith DS, Cottrell J, Young W, editors. Anaesthetic management for posterior fossa surgery. Cottrell and Young’s Neuroanesthesia. Philadelphia PA: Mosby Elsevier; 2010. p. 203-17