- Departments of Anesthesiology and Intensive Care Medicine, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Rome.
- Departments of Neurosurgery, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Rome.
Correspondence Address:
Nicola Montano
Departments of Neurosurgery, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome, Rome.
DOI:10.25259/SNI_552_2019
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Eleonora Ioannoni, Giuseppe Grande, Alessandro Olivi, Massimo Antonelli, Anselmo Caricato, Nicola Montano. Factors affecting serum lactate in patients with intracranial tumors – A report of our series and review of the literature. 06-Mar-2020;11:39
How to cite this URL: Eleonora Ioannoni, Giuseppe Grande, Alessandro Olivi, Massimo Antonelli, Anselmo Caricato, Nicola Montano. Factors affecting serum lactate in patients with intracranial tumors – A report of our series and review of the literature. 06-Mar-2020;11:39. Available from: https://surgicalneurologyint.com/surgicalint-articles/9891/
Abstract
Background: A hyperlactemia may occur in the presence of tissue hypoperfusion, in diseases affecting metabolism and in cases of malignant neoplasm. However, the factors affecting the serum lactate levels in patients submitted to craniotomy for the resection of an intracranial tumor have been investigated only marginally. Here, we assessed the factors possibly affecting the levels of serum lactate in intracranial tumors and carried out a thorough literature review on this topic.
Methods: All patients submitted to elective craniotomy from January 2017 to August 2018 for the resection of a glioblastoma (GBM; 101 cases) and a benign meningioma (WHO I; 105 cases) were included in this study. The sex, age, histological diagnosis, body mass index (BMI), and diabetes were assessed as possible factors affecting the level of the preoperative and postoperative serum lactate in these patients.
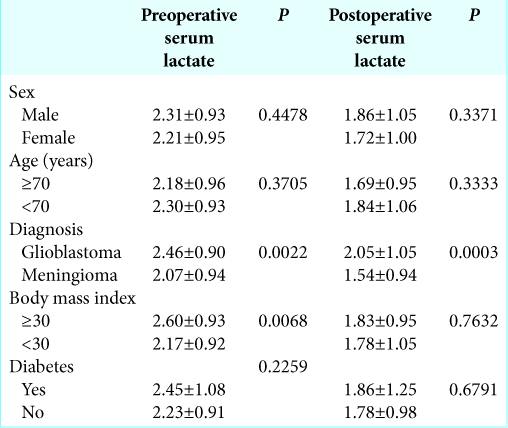
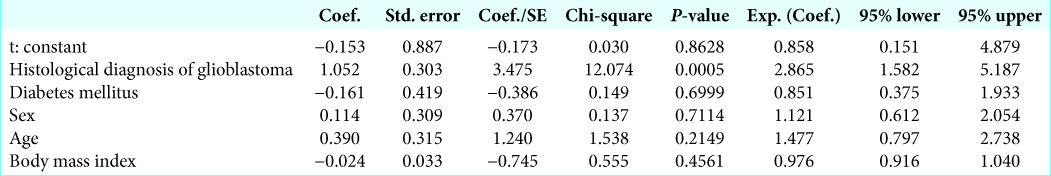
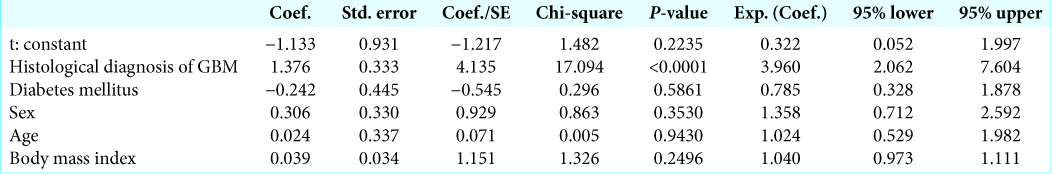
Results: We found that preoperative hyperlactemia (> 2 mmol/l) was more frequent in patients with GBM than in patients with meningioma (P = 0.0003). Moreover, a strong correlation between a preoperative lactemia and postoperative lactemia (P P = 0.0022) and in patients with a BMI ≥30 (P = 0.0068). Postoperative serum lactate levels were significantly higher in GBM patients (P = 0.0003). On multivariate logistic regression analysis, a diagnosis of GBM was an independent factor for higher level of preoperative (P = 0.0005) and postoperative (P
Conclusion: The malignant phenotype of GBM is the strongest factor associated with a pre- and postoperative hyperlactemia in patients submitted to craniotomy for the resection of an intracranial tumor.
Keywords: Brain tumor metabolism, Craniotomy, Glioblastoma, Meningioma, Serum lactate
INTRODUCTION
Serum lactate, an end product of anaerobic metabolism, is used as an indicator of poor tissue perfusion and a measure of illness severity. Two types of hyperlactemia have been described. Type A is seen in acute care settings, with a serum lactate >4 mmol/L and metabolic acidosis, along with clinical signs of tissue hypoperfusion. This kind of hyperlactemia has been associated with higher mortality in that clinical context.[
The aim of this study was to assess the factors possibly affecting the levels of serum lactate in intracranial tumors. Moreover, we also reviewed the pertinent literature.
MATERIALS AND METHODS
In this retrospective observational study, we included all patients submitted to elective craniotomy for the resection of a glioblastoma (GBM) and a benign meningioma (WHO I) consecutively admitted to our neurointensive care unit from January 2017 to August 2018. Exclusion criteria were considered age <18 and >80, and all known factors involved in hyperlactemia, such as sepsis, shock, hepatic and renal failure, catecholamine infusion, and antiretroviral drugs. Serum lactate levels were evaluated immediately before anesthesia induction and on admission in neurointensive care unit after surgery by enzymatic method (normal ranges 0.7–2.0 mmol/L; Nova Biomedical, Stat Profile CCX, Waltham, MA, USA). A formal ethical approval was not required by local Ethical Committee due to retrospective and observational nature of the study. Sex, age (<70 vs. ≥70 years), histological diagnosis, body mass index (BMI) (<30 vs. ≥30), and diabetes were assessed as possible factors affecting the level of the preoperative and postoperative serum lactate. Statistical comparison of continuous variables was performed by the Student’s t-test. Statistical comparison of categorical variables was performed by Chi-square statistic. A multivariate logistic regression model was used to estimate the odds ratio to have a high serum lactate levels (> 2 mmol/l) in the preoperative and postoperative, while adjusting for baseline variables that included sex, age (<70 vs. ≥70 years), histological diagnosis, BMI (<30 vs. ≥30), and the presence of a history of diabetes. Differences were considered significant at P < 0.05. Statistical analyses were conducted using StatView version 5 software (SAS Institute Inc.).
RESULTS
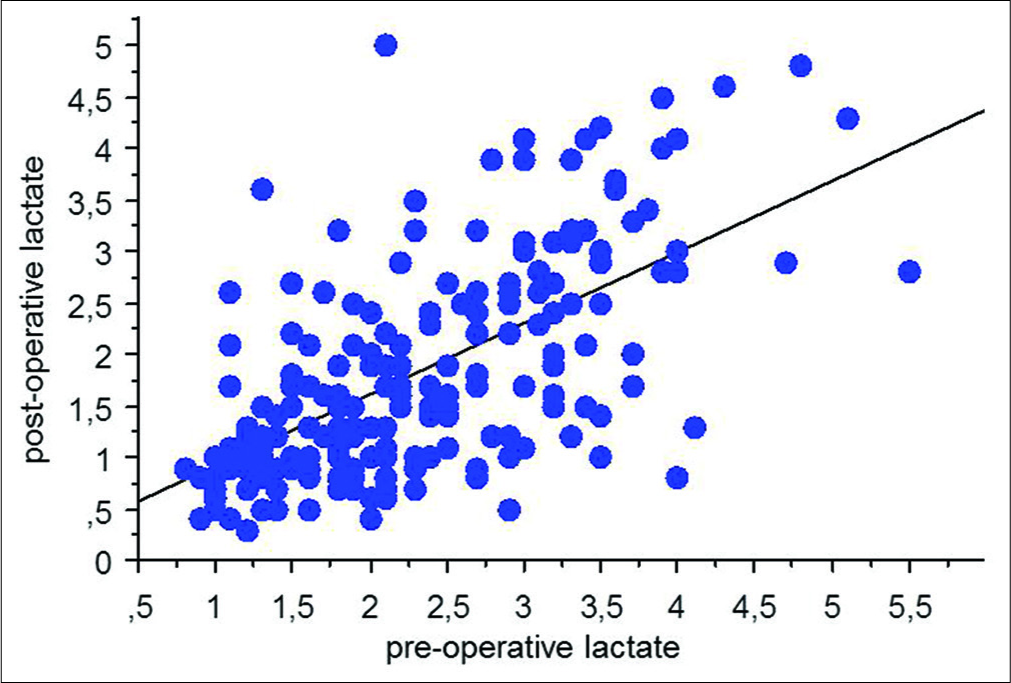
We observed 101 patients with primary GBM (63 males and 37 females) and 105 patients with benign meningioma (39 males and 66 females). The mean age was 63.2 ± 16.9 years in GBM group and 61.0 ± 14.4 in meningioma group, respectively. Preoperative hyperlactemia (> 2 mmol/l) was more frequent in patients with GBM than in patients with meningioma (65.34% in patients with GBM and 40.00% in patients with meningioma; P = 0.0003, Fisher’s exact test). A strong correlation between preoperative lactemia and postoperative lactemia was found (P < 0.0001, Spearman rank correlation;
DISCUSSION
The main results of this study were the following: (1) hyperlactemia is more frequent in GBM, the most malignant brain tumor than in benign meningioma; (2) preoperative lactemia and postoperative lactemia are strictly related; and (3) the histological diagnosis of GBM is the only independent factor significantly associated with pre- and post-operative hyperlactemia.
GBM is the most frequent and most malignant central nervous system (CNS) tumor with an incidence of 3.9 cases/100,000/year. It represents 65–70% of astrocytic tumors and 12–15% of CNS neoplasms with a classical incidence peak between 55 and 74 years.[
The main strength of our study is that trough a rigorous statistical analysis, we demonstrated that the main factor involved in the hyperlactemia in patients submitted to craniotomy is the malignant phenotype of the tumor and this can help the anesthesiologist and the neurointensivist in correctly managing these patients. Moreover, we carried out an extensive review of the literature on this topic. On the other hand, our study has some limitations such as the lack of a correlation with the survival of these patients. This is an important topic to address in future although the previous studies failed to demonstrate a correlation between increased serum lactate and patient’s prognosis.[
CONCLUSION
Our data and the literature review showed that the malignant phenotype of GBM is the strongest factor determining the pre- and post-operative hyperlactemia in patients submitted to craniotomy for the resection of an intracranial tumor. This should always kept in mind in the intraoperative and postoperative management of these patients.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
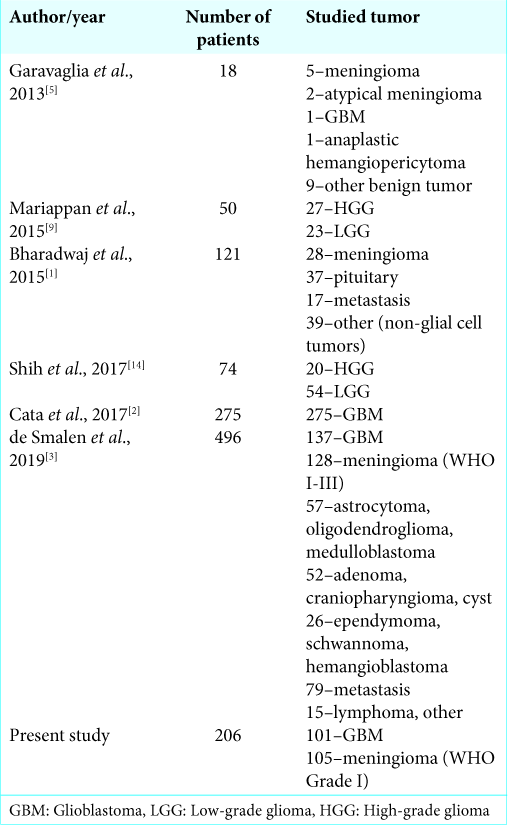
1. Bharadwaj S, Venkatraghavan L, Mariappan R, Ebinu J, Meng Y, Khan O. Serum lactate as a potential biomarker of non-glial brain tumors. J Clin Neurosci. 2015. 22: 1625-7
2. Cata JP, Bhavsar S, Hagan KB, Arunkumar R, Grasu R, Dang A. Intraoperative serum lactate is not a predictor of survival after glioblastoma surgery. J Clin Neurosci. 2017. p. 43224-8
3. de Smalen PP, van Ark TJ, Stolker RJ, Vincent AJ, Klimek M. Hyperlactatemia after intracranial tumor surgery does not affect 6-month survival: A retrospective case series. J Neurosurg Anesthesiol. 2020. 32: 48-56
4. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012. 14: v1-49
5. Garavaglia M, Mak T, Cusimano MD, Rigamonti A, Crescini C, McCredy V. Body mass index as a risk factor for increased serum lactate during craniotomy. Minerva Anestesiol. 2013. 79: 1132-9
6. Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis A retrospective outcome evaluation of critically ill patients. Crit Care. 2006. 10: R22-
7. Liberti MV, Locasale JW. The Warburg effect: How does it benefit cancer cells?. Trends Biochem Sci. 2016. 41: 211-8
8. Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015. 356: 156-64
9. Mariappan R, Venkatraghavan L, Vertanian A, Agnihotri S, Cynthia S, Reyhani S. Serum lactate as a potential biomarker of malignancy in primary adult brain tumours. J Clin Neurosci. 2015. 22: 144-8
10. Okorie ON, Dellinger P. Lactate: Biomarker and potential therapeutic target. Crit Care Clin. 2011. 27: 299-326
11. Pallini R, Fernandez E, Lauretti L, Doglietto F, D’Alessandris QG, Montano N. Olfactory groove meningioma: Report of 99 cases surgically treated at the Catholic University school of medicine, Rome. World Neurosurg. 2015. 83: 219-310
12. Pereira BJ, Oba-Shinjo SM, de Almeida AN, Marie SK. Molecular alterations in meningiomas Literature review. Clin Neurol Neurosurg. 2019. p. 17689-96
13. Phypers B, Pierce JM. Lactate physiology in health and disease. Contin Educ Anaesth Crit Care Pain. 2006. 6: 128-32
14. Shih CC, Lee TS, Tsuang FY, Lin PL, Cheng YJ, Cheng HL. Pretreatment serum lactate level as a prognostic biomarker in patients undergoing supratentorial primary brain tumor resection. Oncotarget. 2017. 8: 63715-23
15. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
16. Tennant DA, Durán RV, Boulahbel H, Gottlieb E. Metabolic transformation in cancer. Carcinogenesis. 2009. 30: 1269-80
17. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009. 324: 1029-33






Beathe Sitter
Posted April 9, 2021, 7:52 am
Dear Dr. Montano and co-authors
I have read your paper on serum lactate in patients with intracranial tumors with great interest, and hope you would elaborate on the following:
Figure 1 demonstrates a strong linearity between pre- and post-surgery serum lactate levels, and you suggest that this seems to exclude the hypothesis that hyperlactatemia could be related to systemic hypoperfusion or to the surgical manipulation of the brain.
It has been suggested that hyperlactatemia in patients with brain tumors is caused by lactate production by the cancer cells. If cancer cells were the main cause for elevated serum lactate, surgical removal of the tumor should bring an end to the excess production of lactate. Also, I would assume this effect on serum lactate to be quite immediate. This should show as a substantial drop in post-surgery serum lactate for patients with pre-surgery hyperlactatemia. But Figure 1 shows just a small reduction in serum lactate from pre- to post-surgery. And for all patients, not just patients with hyperlactatemia. From this, I would suggest that your data indicates that the elevated pre-surgery serum lactate is not caused by cancer cells.
I hope that you would elaborate on this matter.