- Department of Neurosurgery, Salford Royal Hospital, Salford, Manchester, United Kingdom.
Correspondence Address:
Damilola Alexander Jesuyajolu, Department of Neurosurgery, Salford Royal Hospital, Salford, Manchester, United Kingdom.
DOI:10.25259/SNI_1121_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Damilola Alexander Jesuyajolu, Terngu Moti, Abdulahi Ajape Zubair, Adnan Alnaser, Ahmed Zanaty, Tom Grundy, Julian Evans. Factors associated with post traumatic hydrocephalus following decompressive craniectomy: A single-center experience. 20-Jan-2023;14:18
How to cite this URL: Damilola Alexander Jesuyajolu, Terngu Moti, Abdulahi Ajape Zubair, Adnan Alnaser, Ahmed Zanaty, Tom Grundy, Julian Evans. Factors associated with post traumatic hydrocephalus following decompressive craniectomy: A single-center experience. 20-Jan-2023;14:18. Available from: https://surgicalneurologyint.com/surgicalint-articles/12113/
Abstract
Background: A decompressive craniectomy (DC) is a surgical procedure sometimes utilized to manage refractory intracranial hypertension following severe traumatic brain injury (sTBI). The previous studies have established a relationship between DC and post traumatic hydrocephalus (PTH). This study aimed to identify the factors responsible for developing shunt-amenable PTH in patients who underwent DC following sTBI.
Methods: A review of a prospectively collected database of all patients admitted with severe TBI in a tertiary neurosurgical center in North-west England between January 2012 and May 2022 was performed. PTH was defined as evidence of progressive ventricular dilatation, clinical deterioration, and/or the eventual need for cerebrospinal fluid diversion (i.e., a ventriculoperitoneal shunt). Statistical analysis was carried out using IBM SPSS versus 28.0.1.
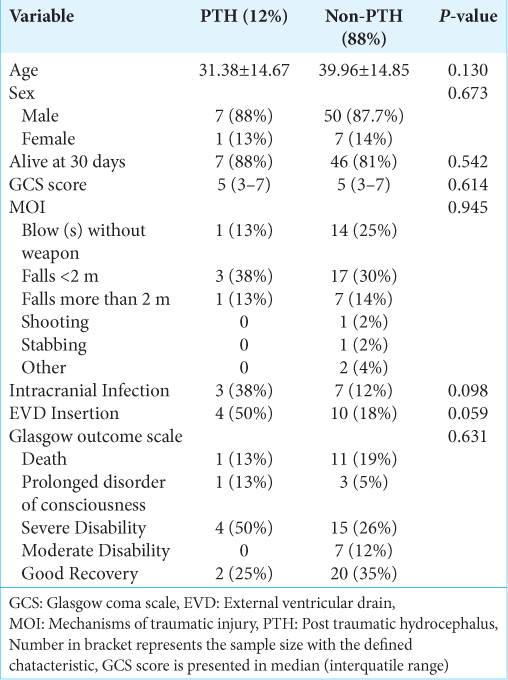
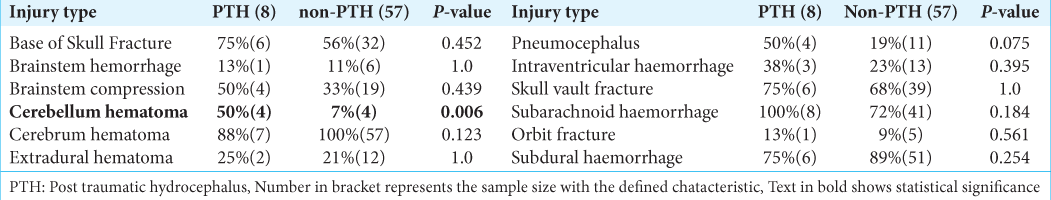
Results: Sixty-five patients met the eligibility criteria and were included in the study. The mean age of the PTH group was 31.38 ± 14.67, while the mean age of the non-PTH group was slightly higher at 39.96 ± 14.85. No statistically significant difference was observed between the two groups’ mechanisms of traumatic injury (P = 0.945). Of the predictors investigated, cerebellar hematoma (and contusions) was significantly associated with PTH (P = 0.006).
Conclusion: This study concludes that cerebellar hematoma (and contusions) are associated with developing PTH in patients undergoing DC.
Keywords: Brain trauma, Severe traumatic brain injury, Trauma, Traumatic brain injury, Ventriculoperitoneal shunt
INTRODUCTION
A decompressive craniectomy (DC) is a surgical procedure that is sometimes deployed to manage refractory intracranial hypertension following severe traumatic brain injury (sTBI).[
The previous studies have established a relationship between DC and PTH.[
MATERIALS AND METHODS
Study population
An audit of a prospectively collected database of all patients admitted with severe TBI in a tertiary neurosurgical center in North-west England was carried out. The project was registered with the Northern Care Alliance Research and Innovation. This included data collected by the local Trauma and Research Network (TARN) from January 2012 to May 2022. We included all patients who had a DC as part of the management of severe TBI. Our exclusion criteria included: Patients who died in the 1st week, patients with incomplete data entries, patients who did not have a DC, and patients who had hydrocephalus before the sTBI. All patients were managed according to the local institutional protocol. Raised intracranial pressure was managed per the measures delineated by the Brain Trauma Foundation Guidelines. As this was a retrospective chart audit, and the data were completely anonymized, patient consent and ethical approval were not required.
Data collection
Datasets were extracted from the local TARN database. This database contains a dataset of prospectively recorded variables covering demographics plus injury-related physiological, investigation, treatment, and outcome parameters that are collated using a standard web-based case record form by the local TARN hospital audit coordinators. Injury descriptions from imaging, operative, and necropsy reports are submitted by TARN coordinators.
Clinical and demographic variables that were extracted from the database included the Glasgow coma scale (GCS) at admission, date of injury, status at 30 days (dead or alive), date of death, no of days from injury to death, date of birth, age, sex, mechanism of injury, DC intervention, type of DC, date of DC, shunt insertion, date of shunt insertion, type of shunt, no of days between injury and shunt insertion, presence of intracranial infection, external ventricular drain (EVD) insertion, the pattern of intracranial injury, and the Glasgow outcome score (GOS). In line with TARN protocol, the GOS was assessed at discharge or death, whichever came first.
Definition of the outcome
PTH was defined as evidence of progressive ventricular dilatation, clinical deterioration and/or the need for CSF diversion (e.g., a ventriculoperitoneal shunt). This definition has been used and established in the previous studies.[
Statistical analysis
Statistical analysis was carried out using IBM SPSS versus 28.0.1. Continuous variables were represented with mean and standard deviations, while categorical variables were expressed as absolute frequencies and percentages. Ordinal variables were represented as medians and IQR. The sample was divided into the PTH and non-PTH groups; statistical analysis was conducted between the two groups to explore statistically significant relationships between different predictors. The student’s t-test was used to evaluate the statistical significance between the mean ages of the PTH group and the non-PTH group. The Wilcoxon Sign-rank test was used to compare the GCS scores and Fisher’s exact test was utilized for the categorical variables. P < 0.05 was considered statistically significant with a 95% confidence interval.
RESULTS
Of the 806 patients reviewed, 65 met the eligibility criteria and were included in the study (PTH group – 8, the nonPTH group – 57). The mean age of the PTH group was 31.38 ± 14.67, while the mean age of the non-PTH group was slightly higher at 39.96 ± 14.85. There were significantly more males in both groups. There was no statistically significant difference between both groups’ age and sex. At 30 days, there was a 12.5% of mortality rate in the PTH group as opposed to 19.3% in the non-PTH group (P = 0.542). The median of the GCS scores was the same (5). No statistically significant difference was observed between the two groups’ mechanisms of traumatic injury (MOI) (P = 0.945). Although a higher percentage of the PTH group (50%) had EVD inserted, it did not reach statistical significance (P = 0.059). Similarly, there was no statistically significant difference between the GOS of the two groups. The summary is shown in
The pattern of intracranial injury was extensively investigated. The different types of injury sustained included the base of skull fracture, brainstem hemorrhage, brainstem compression, cerebellum hematoma, cerebrum hematoma, extradural hematoma, pneumocephalus, intraventricular hemorrhage, skull vault fracture, subarachnoid hemorrhage, orbit fracture, and subdural hemorrhage. Of all the predictors, only cerebellar hematoma (and contusions) had a significant association with PTH (P = 0.006). The summary is shown in
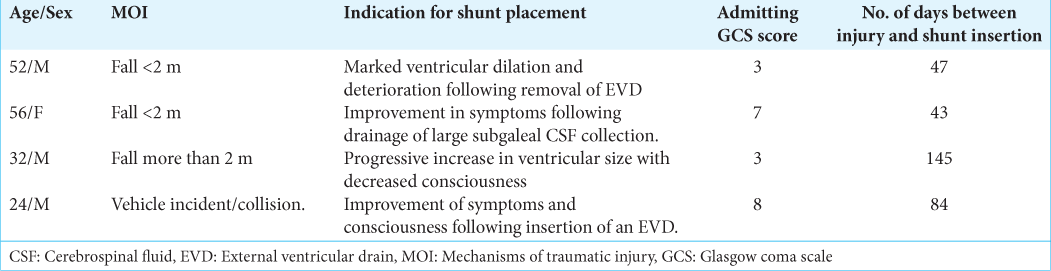
The clinical features of patients who had cerebellar contusions and PTH are also featured in
DISCUSSION
This study primarily focused on identifying clinical predictors of PTH in patients who underwent DC following sTBI. Although DC is a life-saving procedure, identifying the incidence and clinical predictors of PTH in this subset of patients can help inform the counseling and decision-making process. Moreso, it will inform the prognosis during the subsequent neurorehabilitation. Following examination of the factors that could be associated, only the presence of cerebellar contusions demonstrated a statistically significant relationship (P = 0.006). No significant association between the other predictors tested; these included age, sex, the outcome at 30 days, intracranial infection, EVD insertion, MOI, admitting GCS score, and GOS.
Our findings somewhat contrast with other studies investigating this subject. Several significant factors associated with PTH in patients who had DC identified in the literature include admitting GCS <6, intraventricular hemorrhage on the first CT scan, a requirement of bilateral DC,[
Decompressive craniectomies are known to alter the CSF dynamics,[
We experienced some limitations while conducting this investigation. Our definition of the outcome was primarily those who had undergone shunt insertion due to clinically relevant PTH. This could have reduced the number of people said to have PTH in our study. The sample size of this study is also small, which makes our findings to be less generalizable. Further investigation with a larger sample size may be beneficial in validating the findings of this study.
Despite comprehensive data collection, we could not collect data on other factors, including the size of the craniectomy, the distance of the medial edge of the craniectomy from the midline, the length of ICU admission, and the duration of intubation and ventilation. Although we intended to conduct a multivariable analysis of the statistically significant factors in this study, this was not feasible due to the restricted statistical significance of the data. Despite the limitations, we believe that this study will be a valuable addition to the growing body of work on PTH.
CONCLUSION
This series concludes that cerebellar hematoma (and contusions) is associated with developing PTH in patients undergoing DC. This is an important finding for neurosurgeons when considering decompressive craniectomies as a management modality in patients with sTBI.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
We would like to thank the local TARN leads Mr. W.P and Mr. S.H for assisting with material for this audit project.
References
1. Choi I, Park HK, Chang JC, Cho SJ, Choi SK, Byun BJ. Clinical factors for the development of posttraumatic hydrocephalus after decompressive craniectomy. J Korean Neurosurg Soc. 2008. 43: 227-31
2. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011. 364: 1493-502
3. Datar S, Rabinstein AA. Cerebellar hemorrhage. Neurol Clin. 2014. 32: 993-1007
4. Di G, Hu Q, Liu D, Jiang X, Chen J, Liu H. Risk factors predicting posttraumatic hydrocephalus after decompressive craniectomy in traumatic brain injury. World Neurosurg. 2018. 116: e406-13
5. Fotakopoulos G, Tsianaka E, Siasios G, Vagkopoulos K, Fountas K. Posttraumatic hydrocephalus after decompressive craniectomy in 126 patients with severe traumatic brain injury. J Neurol Surg A Cent Eur Neurosurg. 2016. 77: 88-92
6. Goldschmidt E, Deng H, Puccio AM, Okonkwo DO. Post-traumatic hydrocephalus following decompressive hemicraniectomy: Incidence and risk factors in a prospective cohort of severe TBI patients. J Clin Neurosci. 2020. 73: 85-8
7. Guyot LL, Michael DB. Post-traumatic hydrocephalus. Neurol Res. 2000. 22: 25-8
8. Hu Q, Di G, Shao X, Zhou W, Jiang X. Predictors associated with post-traumatic hydrocephalus in patients with head injury undergoing unilateral decompressive craniectomy. Front Neurol. 2018. 9: 337
9. Linnemann M, Tibæk M, Kammersgaard LP. Hydrocephalus during rehabilitation following severe TBI. Relation to recovery, outcome, and length of stay. NeuroRehabilitation. 2014. 35: 755-61
10. Mork J, Mraček J, Štěpánek D, Runt V, Přibáň V. Surgical complications of decompressive craniectomy in patients with head injury. Rozhl Chir. 2020. 99: 316-22
11. Mraček J, Mork J, Dostal J, Tupy R, Mrackova J, Priban V. Complications following decompressive craniectomy. J Neurol Surg A Cent Eur Neurosurg. 2021. 82: 437-45
12. Nasi D, Gladi M, Di Rienzo A, di Somma L, Moriconi E, Iacoangeli M. Risk factors for post-traumatic hydrocephalus following decompressive craniectomy. Acta Neurochir (Wien). 2018. 160: 1691-8
13. Rufus P, Moorthy RK, Joseph M, Rajshekhar V. Post traumatic hydrocephalus: Incidence, pathophysiology and outcomes. Neurol India. 2021. 69: 420-8
14. Shi SS, Zhang GL, Zeng T, Lin YF. Posttraumatic hydrocephalus associated with decompressive cranial defect in severe brain-injured patients. Chin J Traumatol. 2011. 14: 343-7
15. Singh R, Pandey N, Singh RC. Traumatic cerebellar hematoma: A tertiary care experience of 23 conservatively managed cases. Asian J Neurosurg. 2020. 15: 882-8
16. Vedantam A, Yamal JM, Hwang H, Robertson CS, Gopinath SP. Factors associated with shunt-dependent hydrocephalus after decompressive craniectomy for traumatic brain injury. J Neurosurg. 2018. 128: 1547-52
17. Yuan Q, Wu X, Yu J, Sun Y, Li Z, Du Z. Subdural hygroma following decompressive craniectomy or non-decompressive craniectomy in patients with traumatic brain injury: Clinical features and risk factors. Brain Inj. 2015. 29: 971-80