- Department of Neurosurgery, Nara Medical University, Kashihara, Japan
Correspondence Address:
Ryosuke Matsuda
Department of Neurosurgery, Nara Medical University, Kashihara, Japan
DOI:10.4103/sni.sni_473_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Koji Omoto, Ryosuke Matsuda, Ichiro Nakagawa, Yasushi Motoyama, Hiroyuki Nakase. False-positive inflammatory change mimicking glioblastoma multiforme under 5-aminolevulinic acid-guided surgery: A case report. 23-Feb-2018;9:49
How to cite this URL: Koji Omoto, Ryosuke Matsuda, Ichiro Nakagawa, Yasushi Motoyama, Hiroyuki Nakase. False-positive inflammatory change mimicking glioblastoma multiforme under 5-aminolevulinic acid-guided surgery: A case report. 23-Feb-2018;9:49. Available from: http://surgicalneurologyint.com/surgicalint-articles/false%e2%80%91positive-inflammatory-change-mimicking-glioblastoma-multiforme-under-5%e2%80%91aminolevulinic-acid%e2%80%91guided-surgery-a-case-report/
Abstract
Background:5-aminolevulinic acid (5-ALA)–guided surgery is one of the gold standard perioperative modalities for maximum resection of malignant gliomas. However, it should be noted that 5-ALA fluorescence does not definitively indicate the presence of malignant tumor cells.
Case Description:We report a rare case of false-positive lesion mimicking glioblastoma multiforme (GBM) under 5-ALA-guided surgery. A 44-year-old woman presented with persistent headache and flickering in her eyes. Magnetic resonance imaging showed enhanced lesion with perifocal edema in the left occipital lobe. We performed 5-ALA-guided surgery for the lesion, during which strong fluorescence was observed, but evaluation of the intraoperative frozen section revealed only inflammatory cells. We concluded the tumor resection once adequate decompression had been achieved, and made the final pathological diagnosis of inflammatory change following an unknown infection.
Conclusion:Neurosurgeons should be aware of false-positive lesions mimicking GBM under 5-ALA guided surgery.
Keywords: 5-aminolevulinic acid, glioblastoma, inflammatory disease
INTRODUCTION
5-aminolevulinic acid (5-ALA)–guided surgery is one of the gold standard perioperative modalities for maximum resection of malignant gliomas. Recent studies of malignant gliomas have demonstrated that resection under 5-ALA-guided surgery leads to significantly improved progression-free survival[
5-ALA is in itself not fluorescent but serves as the metabolic precursor of heme in the heme biosynthetic pathway where it is metabolized into endogenous fluorescent protoporphyrin IX (PpIX). Consequently, oral intake of 5-ALA results in accumulation of intracellular PpIX. Exposure to blue light at 405 nm wavelength causes excitation of the PpIX, allowing it to become visible as red fluorescence. Because 5-ALA–guided surgery depends not on the tumor itself but rather on the fluorescence of accumulated intracellular PpIX, neurosurgeons should be aware of false-positive findings that can result in higher accumulations of PpIX. For example, false-positives have occurred with radiation necrosis,[
CASE DESCRIPTION
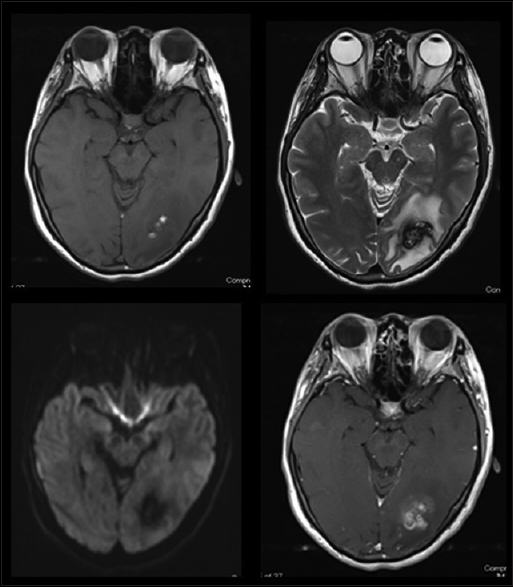
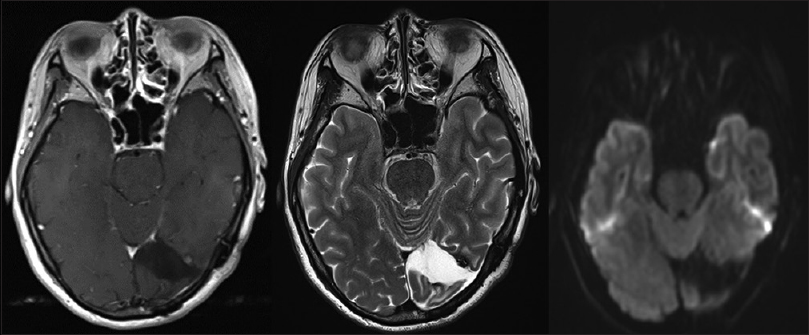
A 44-year-old woman with no history presented at a local hospital with persistent headache and flickering in her eyes. Computed tomography (CT) of the head revealed a small amount of hemorrhage with strong perifocal edema in the left occipital lobe. She was eventually transferred to our hospital for further treatment. Contrast-enhanced magnetic resonance imaging (MRI) showed heterogeneous enhancement in the left occipital lobe [
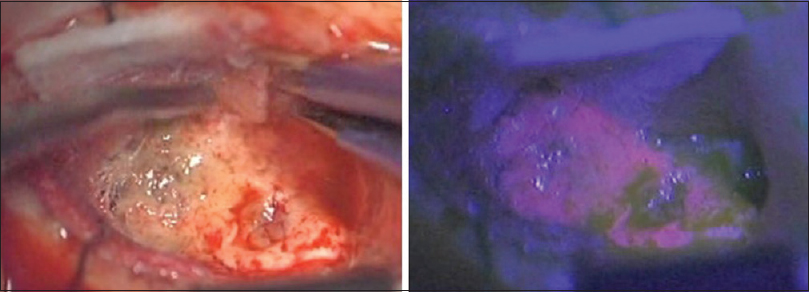
We performed a lesionectomy with enhanced lesion to confirm the pathological diagnosis and reduce edema in the brain. We did not resect the peritumoral lesion despite the vague fluorescence. After the surgical resection, the patient exhibited only right upper quadrant hemianopia. Histopathological examination with hematoxylin-eosin (HE) staining revealed [
Figure 3
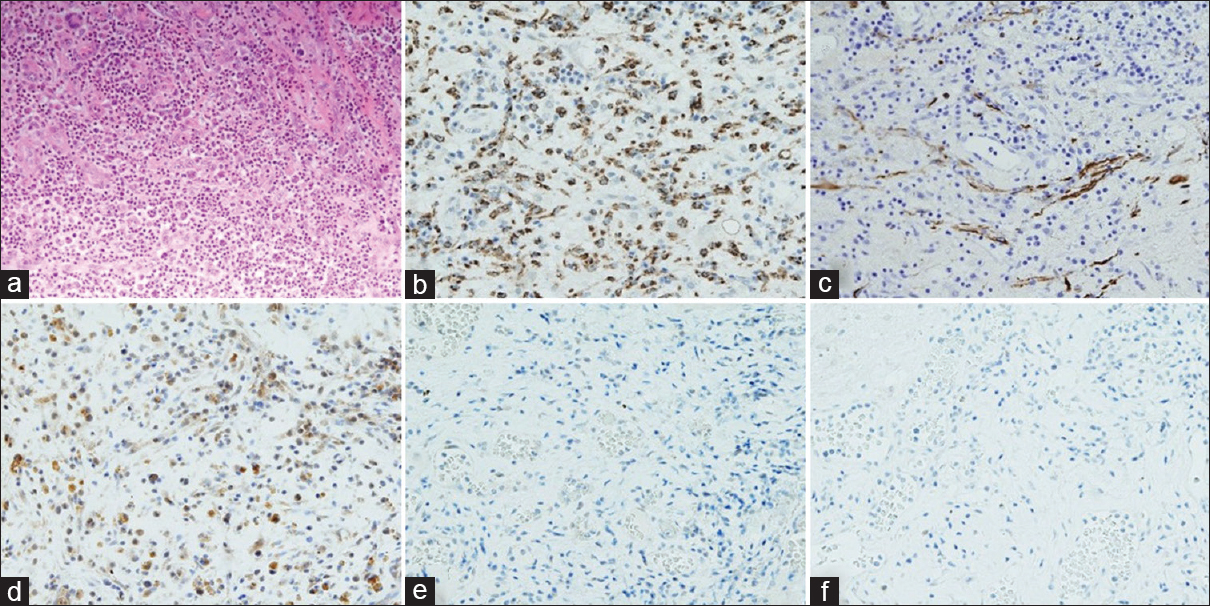
HE stain and immunohistochemistry (a) HE, (b) CD68, (c) GFAP, (d) PEPT1, (e) ABCG2, (f) FECH. All of them are ×200. HE stain shows aggregation of macrophages and lymphocytes and no tumor cells because of high expression of CD68 that prove the presence of macrophages and normal expression of GFAP that prove no tumor cells. High expression of PEPT1 and no expression of ABCG2 and FECH indicates increased accumulation of intracellular PpIX
DISCUSSION
5-ALA–guided surgery for resection of malignant gliomas is a standard surgical procedure at many neuro-oncological centers. Therefore, it is crucial for neurosurgeons to understand the mechanisms involved in intraoperative 5-ALA fluorescence. GBM is a locally invasive tumor that has poor prognosis despite treatment. The outcomes for patients with GBM can vary depending on the individual characteristics of the patient and the specific tumor. Patient age, Karnofsky Performance Status (KPS) score, and extent of resection are the most significant clinical predictors of survival for patients with GBM.[
However, it should be noted that 5-ALA fluorescence does not definitively indicate the presence of malignant tumor cells. Researchers have reported cases of nontumorous conditions mimicking GBM by displaying high 5-ALA fluorescence intraoperatively, such as radiation necrosis, multiple sclerosis, abscess, and cerebral infarction.[
5-ALA is a precursor molecule in the heme biosynthetic pathway, where its metabolism induces the production of PpIX. Ferrous iron is added to PpIX, a reaction catalyzed by FECH, to form nonfluorescent heme. The previous study mentioned that FECH has been known to cause enzyme-mediated intracellular accumulation of PpIX in human glioma cells[
In malignant tumor cells, the key enzymes and transporters in the porphyrin-biosynthesis pathway of 5-ALA metabolism are different from those of other types of tumors. However, their presence has been reported with nontumorous disease. Most likely, the mechanisms responsible for fluorescence in nontumorous conditions are distinct activated enzyme or transporter expression patterns associated with porphyrin-biosynthesis pathways similar to those of tumor cells.
In our patient, the occurrence of intraoperative fluorescence can be explained by the increased expression of PEPT1 and the suppressed expression of ABCG2 and FECH found with IHC. This reaction may be caused by the presence of an infection, which triggers an immune response leading to an invasion of microglia, neutrophils, macrophages, and lymphocytes. Macrophages, activated lymphocytes, and neutrophils cause increased accumulation of PpIX when incubated with 5-ALA.[
CONCLUSION
This case report presents the first description of 5-ALA–induced fluorescence with IHC caused by inflammatory change after an unknown infection. 5-ALA–guided surgery is a useful modality for the removal of brain tumors. However, false-positives can occur with accumulation of PpIX, as in our patient, which can lead to extensive removal and risk of critical postoperative complications. It is important to use caution with 5-ALA-guided surgery for tumor resection.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aldave G, Tejada S, Pay E, Marigil M, Bejarano B, Idoate MA. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery. 2013. 72: 915-20
2. Behling F, Hennersdorf F, Bornemann A, Tatagiba M, Skardelly M. 5-aminolevulinic acid accumulation in a cerebral infarction mimicking high-grade glioma. World Neurosurg. 2016. 92: 585-8
3. Bissonnette R, Zeng H, McLean DI, Korbelik M, Lui H. Oral aminolevulinic acid induces protoporphyrin ix fluorescence in psoriatic plaques and peripheral blood cells. Photochem Photobiol. 2001. 74: 339-45
4. Fujino M, Nishio Y, Ito H, Tanaka T, Li XK. 5-aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int Immunopharmacol. 2016. 37: 71-8
5. Hagiya Y, Endo Y, Yonemura Y, Takahashi K, Ishizuka M, Abe F. Pivotal roles of peptide transporter pept1 and atp-binding cassette (abc) transporter abcg2 in 5-aminolevulinic acid (ala)-based photocytotoxicity of gastric cancer cells in vitro . Photodiagnosis Photodyn Ther. 2012. 9: 204-14
6. Ishikawa T, Kajimoto Y, Inoue Y, Ikegami Y, Kuroiwa T. Critical role of abcg2 in ala-photodynamic diagnosis and therapy of human brain tumor. Adv Cancer Res. 2015. 125: 197-216
7. Kamp MA, Felsberg J, Sadat H, Kuzibaev J, Steiger HJ, Rapp M. 5-ala-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir (Wien). 2015. 157: 207-13
8. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
9. Miyake M, Ishii M, Kawashima K, Kodama T, Sugano K, Fujimoto K. siRNA-mediated knockdown of the heme synthesis and degradation pathways: Modulation of treatment effect of 5-aminolevulinic acid-based photodynamic therapy in urothelial cancer cell lines. Photochem Photobiol. 2009. 85: 1020-7
10. Miyatake S, Kuroiwa T, Kajimoto Y, Miyashita M, Tanaka H, Tsuji M. Fluorescence of non-neoplastic, magnetic resonance imaging-enhancing tissue by 5-aminolevulinic acid: Case report. Neurosurgery. 2007. 61: 1101-3
11. Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: A phase ii study. Neurosurgery. 2009. 65: 1070-6
12. Rittenhouse-Diakun K, Van Leengoed H, Morgan J, Hryhorenko E, Paszkiewicz G, Whitaker JE. The role of transferrin receptor (cd71) in photodynamic therapy of activated and malignant lymphocytes using the heme precursor delta-aminolevulinic acid (ala). Photochem Photobiol. 1995. 61: 523-8
13. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011. 115: 3-8
14. Sharma S, Jajoo A, Dube A. 5-aminolevulinic acid-induced protoporphyrin-ix accumulation and associated phototoxicity in macrophages and oral cancer cell lines. J Photochem Photobiol. 2007. 88: 156-62
15. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase iii trial. Lancet Oncol. 2006. 7: 392-401
16. Teng L, Nakada M, Zhao SG, Endo Y, Furuyama N, Nambu E. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer. 2011. 104: 798-807
17. Voellger B, Klein J, Mawrin C, Firsching R. 5-aminolevulinic acid (5-ala) fluorescence in infectious disease of the brain. Acta Neurochir (Wien). 2014. 156: 1977-8