- Department of Neurosurgery, University of Tsukuba, Japan.
- Department of Neurosurgery, Tsukuba Medical Center Hospital, Tsukuba, Ibaraki, Japan.
Correspondence Address:
Eiichi Ishikawa, Department of Neurosurgery, University of Tsukuba, Tsukuba, Ibaraki, Japan.
DOI:10.25259/SNI_384_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Akinari Yamano1, Yasunobu Nakai2, Kazuki Akutagawa1, Haruki Igarashi2, Kazuaki Tsukada1, Toshitsugu Terakado1, Kazuya Uemura2, Eiichi Ishikawa1. Fatal recurrent ischemic stroke caused by vertebral artery stump syndrome. 06-Sep-2021;12:445
How to cite this URL: Akinari Yamano1, Yasunobu Nakai2, Kazuki Akutagawa1, Haruki Igarashi2, Kazuaki Tsukada1, Toshitsugu Terakado1, Kazuya Uemura2, Eiichi Ishikawa1. Fatal recurrent ischemic stroke caused by vertebral artery stump syndrome. 06-Sep-2021;12:445. Available from: https://surgicalneurologyint.com/surgicalint-articles/11096/
Abstract
Background: Vertebral artery stump syndrome (VASS) develops into recurrent posterior circulation ischemic stroke after ipsilateral vertebral artery (VA) occlusion at its origin.
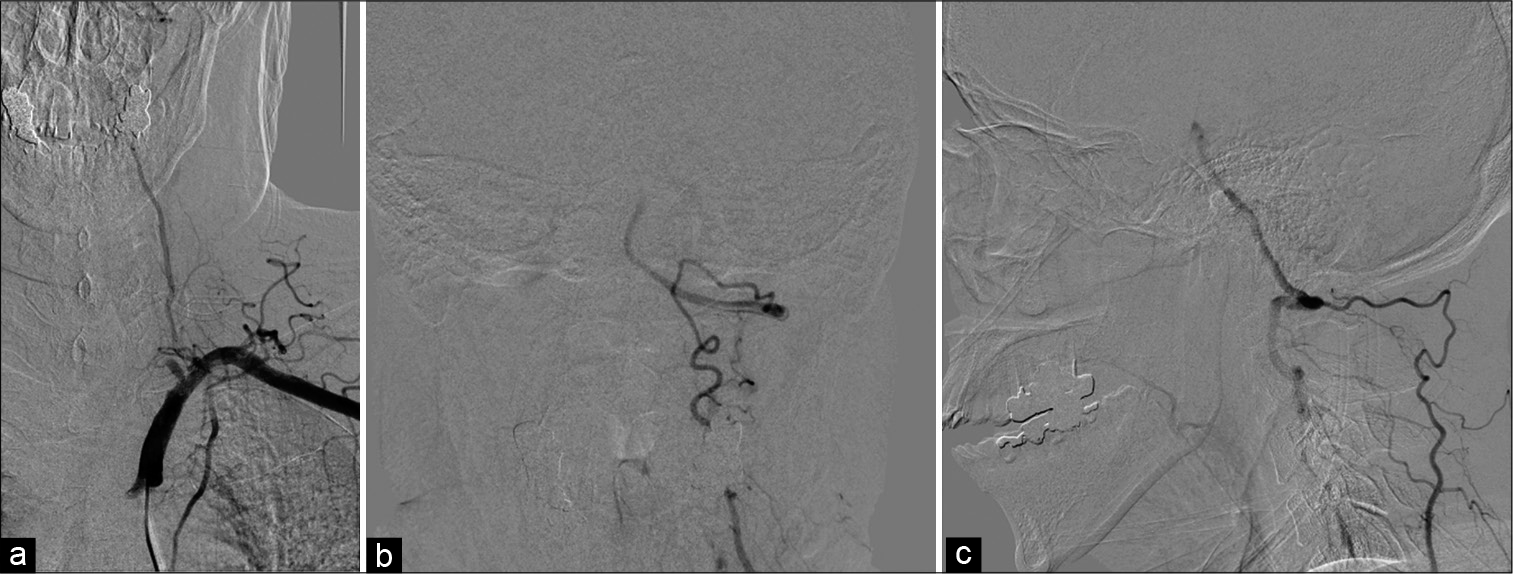
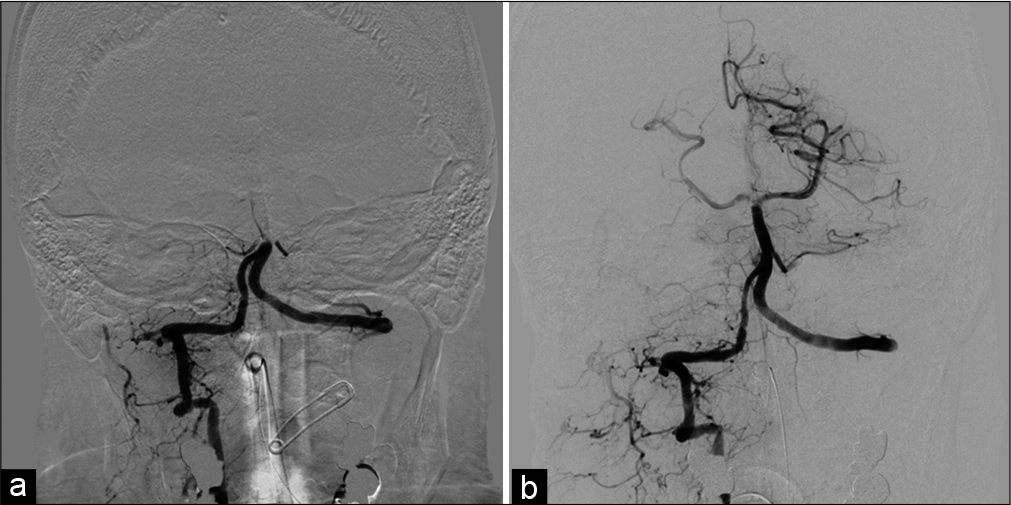
Case Description: The patient was a 46-year-old man with the right posterior cerebral artery occlusion. We used a recombinant tissue plasminogen activator (rt-PA) and then performed mechanical thrombectomy using a stent retriever. Angiography revealed left VA occlusion and stagnant flow to the left VA from the right deep cervical artery; therefore, we diagnosed VASS. Within 24 h of the rt-PA injection, the symptoms had dramatically improved, and so we avoided additional antithrombotic agents. Only 13 h later, the patient developed a basilar artery occlusion and died in spite of a repeated mechanical thrombectomy.
Conclusion: Vigilance against early (and sometimes fatal) recurrent stroke induced by VASS is required.
Keywords: Endovascular treatment, Ischemic stroke, Stroke, Vertebral artery stump syndrome
INTRODUCTION
Vertebral artery stump syndrome (VASS) presents with recurrent posterior circulation (PC) stroke after ipsilateral vertebral artery (VA) occlusion at its origin.[
CASE DESCRIPTION
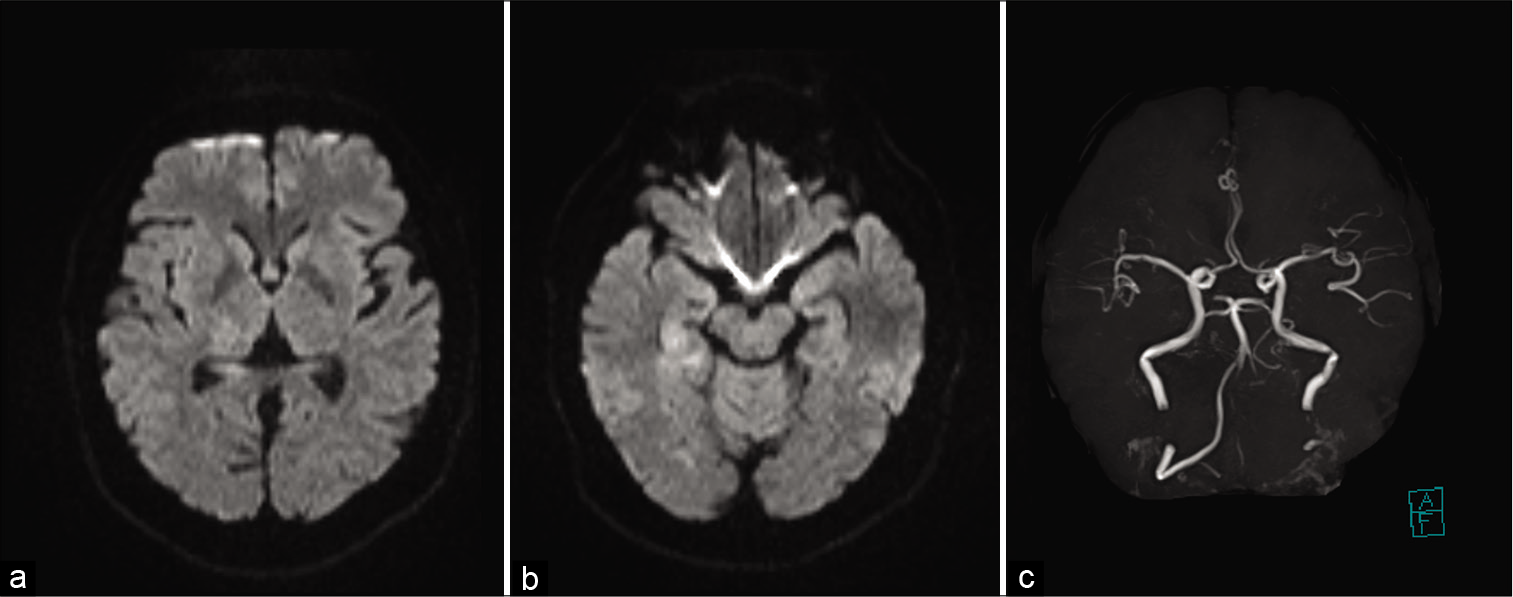
A 46-year-old man arrived at our emergency room with sudden, disturbed consciousness, left homonymous hemianopsia, left hemiplegia, and left hypoesthesia. The National Institutes of Health Stroke Scale (NIHSS) score was 10. Magnetic resonance (MR) diffusion-weighted imaging revealed slightly high-intensity signals in the right occipital lobe, right thalamus, and right hippocampus [
Figure 3:
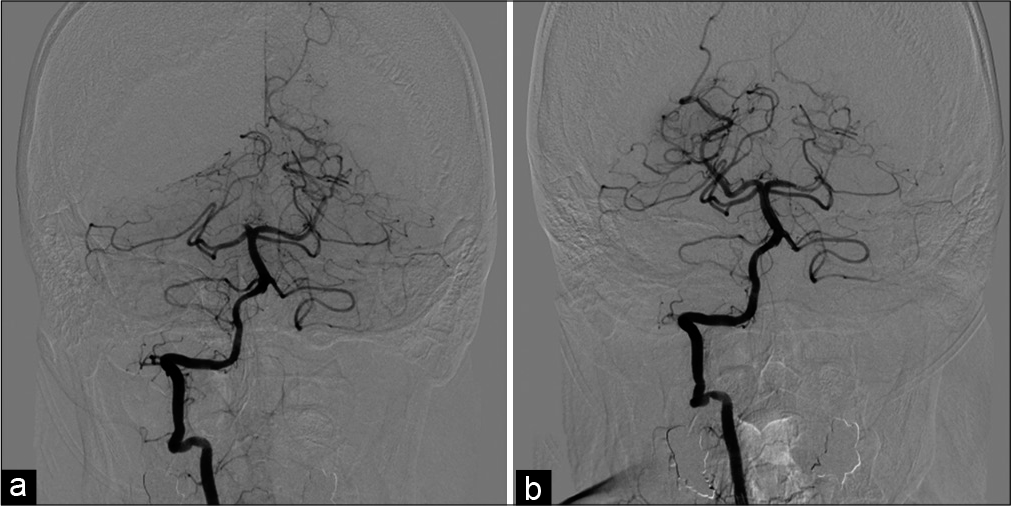
Angiography of the left subclavian artery. The left VA was occluded from its origin (a). Anterior-posterior view (b) and lateral view (c) of angiography of the left subclavian artery. The left deep cervical artery was anastomosed to the left VA and its antegrade flow in the VA was stagnant. VA: Vertebral artery.
DISCUSSION
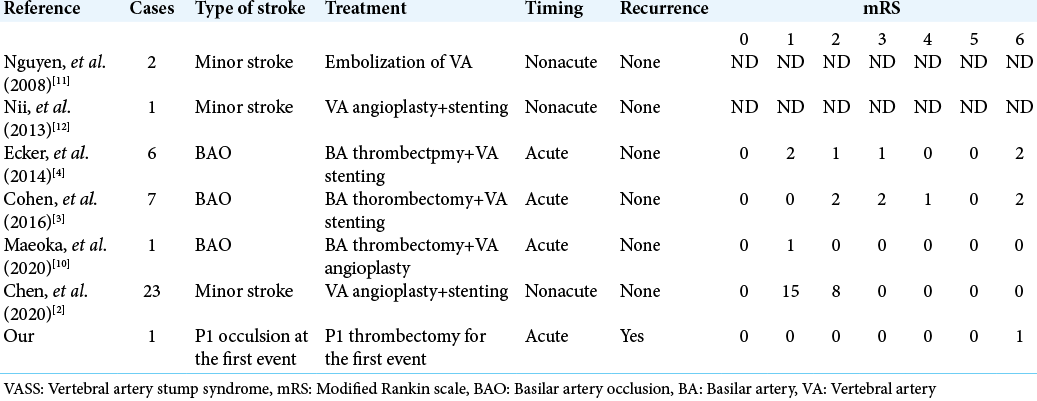
In 2008, Nguyen et al. reported two cases of recurrent PC stroke due to VA origin occlusion, calling it “VASS” because of the clinical similarities to carotid stump syndrome.[
Intravenous rt-PA is known as an effective treatment for acute ischemic stroke[
Endovascular treatment is reported to permanently prevent recurrent stroke, as seen when Nguyen et al. reported two cases of VASS treated with coil embolization to exclude the embolic source at the VA.[
There are several reports of endovascular treatment for BAO due to VASS.[
In our case, the stagnant flow at the VA continued to produce new thrombi despite IV rt-PA use. Because the volume from the VA origin to the anastomosis with the collaterals was relatively large, the stagnant flow had enough space to produce a large thrombus, which had the potential to occlude the BA. The possibility of recurrent stroke, including fatal BA occlusion, must be considered, especially in the acute phase, if antithrombotic therapy is withheld. Even though VASS is not a rare cause of PC stroke, only a few cases have been reported. The concept of VASS must be spread for the sake of accurate diagnosis and to accumulate case numbers sufficient for evaluating the efficacy and safety of early use of antithrombotic and/or radical endovascular treatment.
CONCLUSION
In patients with PC ischemic stroke featuring VA occlusion, VASS, as a dangerous syndrome involving the risk of fatal recurrent stroke, must be considered as its embolic source. In the present case, fatal BA occlusion developed within 24 h of rt-PA use but administration of rt-PA restricted the early introduction of antithrombotic therapy. Vigilance against such fatal, recurrent strokes must therefore be maintained, particularly when antithrombotic therapy is restricted.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barreto AD, Alexandrov AV, Lyden P, Lee J, Martin-Schild S, Shen L. The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke. 2012. 43: 770-5
2. Chen L, Jiang Y, Hu F, He L, Zheng H. Endovascular revascularization of nonacute symptomatic proximal extracranial vertebral artery occlusion. World Neurosurg. 2020. 134: 39-44
3. Cohen JE, Leker RR, Gomori JM, Eichel R, Rajz G, Moscovici S. Emergent revascularization of acute tandem vertebrobasilar occlusions: Endovascular approaches and technical considerations-Confirming the role of vertebral artery ostium stenosis as a cause of vertebrobasilar stroke. J Clin Neurosci. 2016. 34: 70-6
4. Ecker RD, Tsujiura CA, Baker CB, Cushing D. Endovascular reconstruction of vertebral artery occlusion prior to basilar thrombectomy in a series of six patients presenting with acute symptomatic basilar thrombosis. J Neurointerv Surg. 2014. 6: 379-383
5. Gory B, Eldesouky I, Sivan-Hoffmann R, Rabilloud M, Ong E, Riva R. Outcomes of stent retriever thrombectomy in basilar artery occlusion: An observational study and systematic review. J Neurol Neurosurg Psychiatry. 2016. 87: 520-5
6. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D. Thrombolysis with alteplase 3 to 4. 5 hours after acute ischemic stroke. N Engl J Med. 2008. 359: 1317-29
7. Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ, Lee SH. Stroke outcomes with use of antithrombotics within 24 hours after recanalization treatment. Neurology. 2016. 87: 996-1002
8. Kawano H, Inatomi Y, Hirano T, Yonehara T. Vertebral artery stump syndrome in acute ischemic stroke. J Neurol Sci. 2013. 324: 74-9
9. Kawano H, Inatomi Y, Hirano T, Yonehara T, Uchino M. Anticoagulation therapy for vertebral artery stump syndrome. J Neurol Sci. 2010. 295: 125-7
10. Maeoka R, Nakagawa I, Ohnishi H, Kuga Y, Nakase H, Ohnishi H. A thread of hope for successful revascularization for acute embolic basilar artery occlusion due to miserable vertebral artery stump syndrome. A technical report. J Clin Neurosci. 2020. 73: 299-303
11. Nguyen TN, Raymond J, Mahmoud M, Weill A, Roy D, Guilbert F. Vertebral artery stump syndrome. J Neurol Neurosurg Psychiatry. 2008. 79: 91-2
12. Nii K, Abe G, Iko M, Nomoto Y, Iwae Y, Sakamoto K. Endovascular angioplasty for extracranial vertebral artery occlusion without visualization of the stump of the artery ostium technical note. Neurol Med Chir (Tokyo). 2013. 53: 422-6
13. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2018. 49: e46-110
14. Suzuki M, Dembo T, Hara W, Tajima T, Yamashita M, Oji S. Vertebral artery stump syndrome. Intern Med. 2018. 57: 733-6
15. Tempaku A. Cerebral angiography directly visualizes to-andfro stream of vertebral artery stump syndrome. J Gen Fam Med. 2017. 18: 462-3
16. Von Kummer R, Hache W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke. 1992. 23: 646-52
17. Zinkstok SM, Roos YB. ARTIS Investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: A randomised controlled trial. Lancet. 2012. 380: 731-7