- Department of Residency, Japanese Red Cross Nagahama Hospital, Nagahama, Nagahama, Japan.
- Department of Neurosurgery, Kohka Public Hospital, Kohka, Nagahama, Japan.
- Department of Neurosurgery, Japanese Red Cross Nagahama Hospital, Nagahama, Japan.
Correspondence Address:
Sayaka Ito, Department of Neurosurgery, Kohka Public Hospital, Kohka, Japan.
DOI:10.25259/SNI_427_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Makiko Oomori1, Sayaka Ito2, Kazushi Higuchi3. Fatal ruptured occult arteriovenous malformation in a young adult: An autopsy case report. 01-Jul-2022;13:284
How to cite this URL: Makiko Oomori1, Sayaka Ito2, Kazushi Higuchi3. Fatal ruptured occult arteriovenous malformation in a young adult: An autopsy case report. 01-Jul-2022;13:284. Available from: https://surgicalneurologyint.com/surgicalint-articles/11687/
Abstract
Background: Brain arteriovenous malformations (AVMs) are congenital developmental disorders with unclear causative factors and pathogenic mechanisms. Various epigenetic factors may influence the development and rupture of AVMs. Ruptured AVMs may lead to poor outcomes. Therefore, the risk factors of AVM rupture and treatment strategies for unruptured AVMs should be explored. Herein, we report a case of a fatal ruptured AVM diagnosed by radiological and autopsy findings and review the literature regarding AVM treatment.
Case Description: A 46-year-old man was brought to the hospital with sudden loss of consciousness while sitting on the edge of the bathtub. On examination, he was unconscious with poor breathing efforts. He was intubated and a brain CT scan was performed, which showed an intracerebral hemorrhage (ICH) adjacent to the right trigone with massive intraventricular hemorrhage (IVH) and subarachnoid hemorrhage (SAH). Contrast-enhanced CT scan showed abnormal vessels adjacent to the hematoma. He was diagnosed with ICH associated with IVH and SAH caused by a ruptured abnormal vascular lesion. He underwent external ventricular drainage to control the intracranial pressure. He remained unconscious and died 16 h after hospital admission. Autopsy was performed to identify the cause of ICH. Pathological sections showed a mass of blood vessels, measuring 20 × 10 × 10 mm in size, within the hematoma with a single drainer connecting to the transverse sinus. These blood vessels had variable size, shape, and wall thickness on microscopy. Some vessels had abnormal thickened walls with discontinuous elastic fibers. Based on the radiological and autopsy findings, an ICH secondary to SpetzlerMartin Grade I AVM was confirmed.
Conclusion: If the cause of ICH cannot be determined during a patient’s life, autopsy may be performed to determine the pathophysiology of occult vascular lesions, including AVMs. Patients with AVMs may have moderate or no symptoms before and after rupture. Because deep AVMs fed by posterior circulation have high risk of bleeding, surgical intervention should be considered for these patients to prevent a poor outcome. Low-grade and paraventricular AVMs in a young adult may be successfully treated with multimodal surgery.
Keywords: Diagnostic imaging, Endovascular procedures, Hemorrhagic stroke, Intracranial arteriovenous malformation, Microsurgery
INTRODUCTION
Brain arteriovenous malformations (AVMs) are congenital developmental disorders with unclear causative factors and pathogenic mechanisms. Recently, various epigenetic factors have been identified to influence the development and rupture of AVMs.[
CASE DESCRIPTION
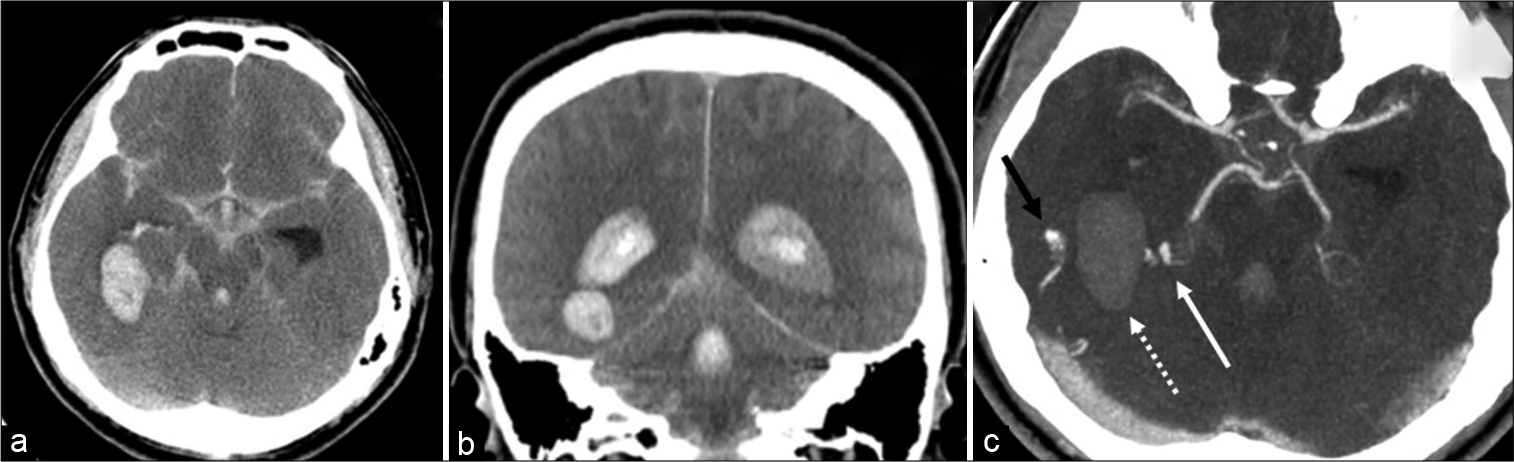
A 46-year-old healthy man was brought to our hospital by ambulance because of sudden loss of consciousness while sitting on the edge of the bathtub. He had no known diseases or history of head trauma. On admission, he was comatose (Glasgow Coma Scale score: 4) with 2 mm round isocoric pupils. He was immediately intubated in the emergency room because of severely suppressed breathing. After achieving hemodynamic stability by administering catecholamine, a brain CT scan was performed, which showed an intracerebral hemorrhage (ICH) adjacent to the right trigone with massive intraventricular hemorrhage (IVH) and subarachnoid hemorrhage (SAH) [
Figure 1:
Brain CT of the patient. Noncontrast CT scan (a: axial and b: coronal views) showing hematoma adjacent to the right trigone, intraventricular hemorrhage, diffuse subarachnoid hemorrhage, edematous brain, and hydrocephalus. Contrast-enhanced CT scan in the axial view (c) showing abnormal vasculature with an artery originating from the right posterior cerebral artery (white arrow), vein draining into the right transverse sinus (black arrow), and hematoma (dotted arrow).
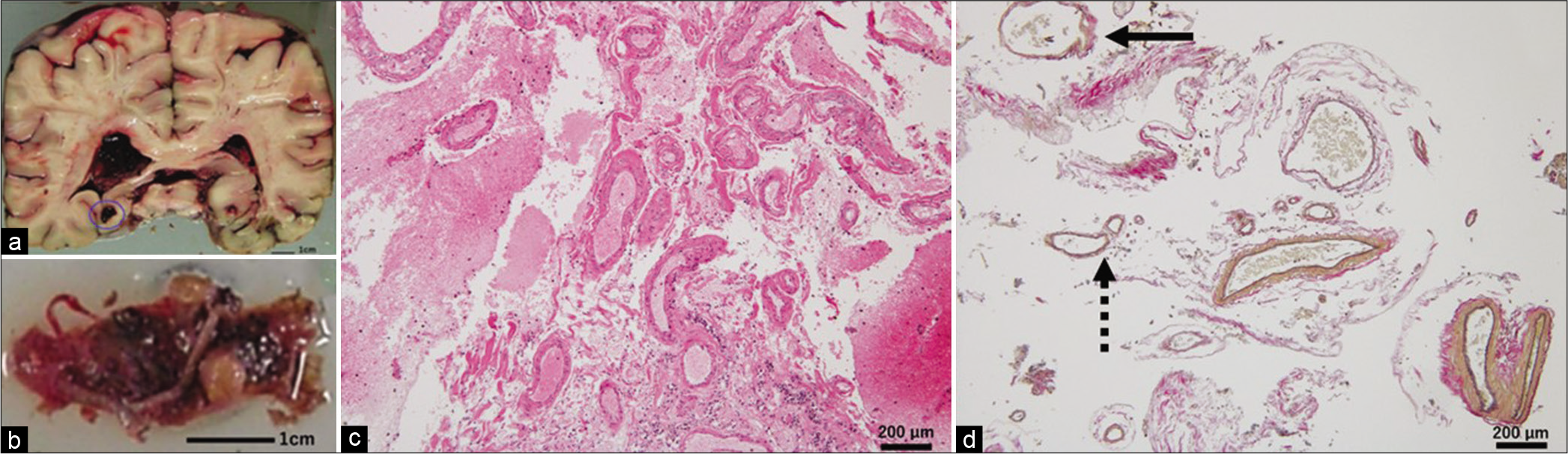
Figure 2:
Pathological findings of the patient. A coronal section of the fixed brain (a) showing subarachnoid hemorrhage at the brain surface and hematoma in the right parahippocampal area although close to the right trigonum (encircled in a), with blood extending into the lateral ventricles. A 20 × 10 × 10 mm mass of blood vessels (b). Staining with hematoxylin and eosin (c) and Elastica van Gieson (d) showing small blood vessels with variable size, shape, and wall thickness. Thin-walled vessels had the appearance similar to veins but contained elastic fibers, which confirmed that they were arteries (dotted arrow in d). Some vessels showed abnormal partially thickened walls with discontinuous elastic fibers (arrow in d).
DISCUSSION
Diagnosis of occult AVM
Angiographically occult vascular malformations are a group of intracranial vascular malformations that cannot be visualized by serial cerebral angiography.[
Our patient had elevated ICP and was hemodynamically unstable; therefore, MRI could not be performed and contrast-enhanced CT scan was performed in the emergency room. Further investigations in our patient, with an MRI or digital subtraction angiography, may have detected the occult AVM.
Occult AVMs may present with subclinical recent or old hemorrhage.[
Histopathological examination of the lesion in our patient showed findings typical of AVMs, that is, many vessels with variable size, shape, and wall thickness, and discontinuous elastic fibers and medial smooth muscle. Compression of the AVM by the hematoma may explain why the AVM was occult. The autopsy of our patient confirmed the diagnosis of occult ruptured AVM with massive IVH and SAH.
Surgical interventions for AVM: microsurgery, endovascular embolization, and radiosurgery
Ruptured AVMs have high morbidity and mortality. The risk factors for AVM rupture are deep location,[
Up to 30% of AVMs are located in the paraventricular region.[
The AVM in our patient was located adjacent to the right trigone, where the pulsatile stress may have predisposed to the hemorrhage. Because of the massive IVH at the initial presentation, our patient did not survive.
In 1961, Margolis et al. reported four patients with ruptured AVMs diagnosed by autopsy. These patients died due to complications of the initial hemorrhage.[
A randomized trial of unruptured brain arteriovenous malformations (ARUBA) was the first randomized and controlled trial to compare medical and surgical treatments for AVM. The surgical interventions included microsurgery, endovascular embolization, and radiosurgery. The trial showed that medical treatment was superior to surgical treatment for preventing stroke and death over follow-up periods of 33 months and 5 years.[
Studies with similar participants and primary endpoints to ARUBA showed that microsurgery is safe and effective for low-grade AVMs with a high cure rate and immediate results;[
In summary, our patient was a 46-year-old adult with a 2 cm sized paraventricular AVM fed by a branch of posterior cerebral artery. The AVM had multiple risk factors for rupture. Unfortunately, the patient presented with massive IVH and did not survive. Patients with AVMs similar to our patient may have moderate or no symptoms before or after rupture. Because these AVMs have high risk of bleeding, surgical intervention should be considered. Low-grade and deep AVMs fed by branch(es) of vertebrobasilar system may be treated by radiosurgery or endovascular embolization with or without microsurgery. Although our patient did not survive, the future patients may be saved.
CONCLUSION
We reported the radiological and autopsy findings of a paraventricular occult AVM with fatal hemorrhage at presentation. If the cause of ICH cannot be determined during a patient’s life, autopsy may be performed. Such investigations may help to determine the pathophysiology of AVMs. Paraventricular AVMs, such as that found in our patient, in a young adult may be successfully treated with multimodal surgery. It is necessary to develop better treatment strategies for patients with AVMs.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
The data that support the findings of this case are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alén JF, Lagares A, Paredes I, Campollo J, Navia P, Ramos A. Cerebral microarteriovenous malformations: A series of 28 cases. J Neurosurg. 2013. 119: 594-602
2. Amin-Hanjani S. ARUBA results are not applicable to all patients with arteriovenous malformation. Stroke. 2014. 45: 1539-40
3. Batjer H, Samson D. Surgical approaches to trigonal arteriovenous malformations. J Neurosurg. 1987. 67: 511-7
4. de Castro-Afonso LH, Vanzim JR, Trivelato FP, Rezende MT, Ulhôa AC, Chodraui-Filho SF. Association between draining vein diameters and intracranial arteriovenous malformation hemorrhage: A multicentric retrospective study. Neuroradiology. 2020. 62: 1497-505
5. Ding D, Starke RM, Kano H, Mathieu D, Huang P, Kondziolka D. Radiosurgery for cerebral arteriovenous malformations in a randomized trial of unruptured brain arteriovenous malformations (ARUBA)-eligible patients: A multicenter study. Stroke. 2016. 47: 342-9
6. Ebeling JD, Tranmer BI, Davis KA, Kindt GW, DeMasters BK. Thrombosed arteriovenous malformations: A type of occult vascular malformation. Magnetic resonance imaging and histopathological correlations. Neurosurgery. 1988. 23: 605-10
7. El-Ghanem M, Kass-Hout T, Kass-Hout O, Alderazi YJ, Amuluru K, Al-Mufti F. Arteriovenous malformations in the pediatric population: Review of the existing literature. Interv Neurol. 2016. 5: 218-25
8. Feghali J, Huang J. Updates in arteriovenous malformation management: The post-ARUBA era. Stroke Vasc Neurol. 2020. 5: 34-9
9. Florian IA, Beni L, Moisoiu V, Timis TL, Florian IS, Balașa A. “De novo” brain AVMs-hypotheses for development and a systematic review of reported cases. Medicina (Kaunas). 2021. 57: 201
10. Friedman WA, Blatt DL, Bova FJ, Buatti JM, Mendenhall WM, Kubilis PS. The risk of hemorrhage after radiosurgery for arteriovenous malformations. J Neurosurg. 1996. 84: 912-9
11. Fuse T, Niwa Y, Umezu M, Yamada K. Growth of occult arteriovenous malformation after cerebral hemorrhage demonstrated by serial magnetic resonance imaging case report. Neurol Med Chir (Tokyo). 2001. 41: 83-6
12. Hartmann A, Stapf C, Hofmeister C, Mohr JP, Sciacca RR, Stein BM. Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke. 2000. 31: 2361-4
13. Hetts SW, Cooke DL, Nelson J, Gupta N, Fullerton H, Amans MR. Influence of patient age on angioarchitecture of brain arteriovenous malformations. AJNR Am J Neuroradiol. 2014. 35: 1376-80
14. Javadpour M, Al-Mahfoudh R, Mitchell PS, Kirollos R. Outcome of microsurgical excision of unruptured brain arteriovenous malformations in ARUBA-eligible patients. Br J Neurosurg. 2016. 30: 619-22
15. Karlsson B, Jokura H, Yang HC, Yamamoto M, Martinez R, Kawagishi J. The NASSAU (New ASSessment of cerebral arteriovenous malformations yet unruptured) analysis: Are the results from the ARUBA trial also applicable to unruptured arteriovenous malformations deemed suitable for gamma knife surgery?. Neurosurgery. 2019. 85: E118-24
16. Kobata H, Kondo A, Iwasaki K, Hattori I. Massive subependymal hemorrhage caused by an occult vascular malformation two case reports. Neurol Med Chir (Tokyo). 1999. 39: 302-7
17. Lang M, Moore NZ, Rasmussen PA, Bain MD. Treatment outcomes of a randomized trial of unruptured brain arteriovenous malformation-eligible unruptured brain arteriovenous malformation patients. Neurosurgery. 2018. 83: 548-55
18. Ma L, Huang Z, Chen XL, Ma J, Liu XJ, Wang H. Periventricular location as a risk factor for hemorrhage and severe clinical presentation in pediatric patients with untreated brain arteriovenous malformations. AJNR Am J Neuroradiol. 2015. 36: 1550-7
19. Margolis G, Odom GL, Woodhall B. Further experiences with small vascular malformations as a cause of massive intracerebral bleeding. J Neuropathol Exp Neurol. 1961. 20: 161-7
20. Marks MP, Lane B, Steinberg GK, Chang PJ. Hemorrhage in intracerebral arteriovenous malformations: Angiographic determinants. Radiology. 1990. 176: 807-13
21. Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997. 350: 1065-8
22. Meyer PG, Orliaguet GA, Zerah M, Charron B, Jarreau MM, Brunelle F. Emergency management of deeply comatose children with acute rupture of cerebral arteriovenous malformations. Can J Anaesth. 2000. 47: 758-66
23. Mohr JP, Overbey JR, Hartmann A, Kummer RV, Al-Shahi Salman R, Kim H. Medical management with interventional therapy versus medical management alone for unruptured brain arteriovenous malformations (ARUBA): Final follow-up of a multicentre, non-blinded, randomised controlled trial. Lancet Neurol. 2020. 19: 573-81
24. Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): A multicentre, non-blinded, randomised trial. Lancet. 2014. 383: 614-21
25. Nerva JD, Mantovani A, Barber J, Kim LJ, Rockhill JK, Hallam DK. Treatment outcomes of unruptured arteriovenous malformations with a subgroup analysis of ARUBA (A randomized trial of unruptured brain arteriovenous malformations)-eligible patients. Neurosurgery. 2015. 76: 563-70
26. Pollock BE, Link MJ, Brown RD. The risk of stroke or clinical impairment after stereotactic radiosurgery for ARUBA-eligible patients. Stroke. 2013. 44: 437-41
27. Rutledge WC, Abla AA, Nelson J, Halbach VV, Kim H, Lawton MT. Treatment and outcomes of ARUBA-eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus. 2014. 37: E8
28. Rutledge WC, Ko NU, Lawton MT, Kim H. Hemorrhage rates and risk factors in the natural history course of brain arteriovenous malformations. Transl Stroke Res. 2014. 5: 538-42
29. Sasaki T, Kurita H, Saito I, Kawamoto S, Nemoto S, Terahara A. Arteriovenous malformations in the basal ganglia and thalamus: Management and results in 101 cases. J Neurosurg. 1998. 88: 285-92
30. Schramm J, Schaller K, Esche J, Boström A. Microsurgery for cerebral arteriovenous malformations: Subgroup outcomes in a consecutive series of 288 cases. J Neurosurg. 2017. 126: 1056-63
31. Shimizu Y, Sasaki K, Ujiie H, Muragaki Y, Kubo O, Hori T. Pathological findings of angiographically occult vascular malformation. J Clin Neurosci. 2002. 9: 19-21
32. Singfer U, Hemelsoet D, Vanlangenhove P, Martens F, Verbeke L, Van Roost D. Unruptured brain arteriovenous malformations: Primary ONYX embolization in ARUBA (a randomized trial of unruptured brain arteriovenous malformations)-eligible patients. Stroke. 2017. 48: 3393-6
33. Stefani MA, Porter PJ, terBrugge KG, Montanera W, Willinsky RA, Wallace MC. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke. 2002. 33: 920-4
34. Thomas JM, Surendran S, Abraham M, Rajavelu A, Kartha CC. Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin Epigenetics. 2016. 8: 78
35. Tonetti DA, Gross BA, Atcheson KM, Jankowitz BT, Kano H, Monaco EA. The benefit of radiosurgery for ARUBA-eligible arteriovenous malformations: A practical analysis over an appropriate follow-up period. J Neurosurg. 2018. 128: 1850-4
36. Tong X, Wu J, Lin F, Cao Y, Zhao Y, Ning B. The effect of age, sex, and lesion location on initial presentation in patients with brain arteriovenous malformations. World Neurosurg. 2016. 87: 598-606
37. Turjman F, Massoud TF, Viñuela F, Sayre JW, Guglielmi G, Duckwiler G. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995. 37: 856-60
38. Verheggen R, Finkenstaedt M, Rittmeyer K, Markakis E. Intra-and paraventricular arteriovenous malformations: Symptomatology, neuroradiological diagnosis, surgical approach and postoperative results. Acta Neurochir (Wien). 1994. 131: 176-83
39. Waga S, Shimosaka S, Kojima T. Arteriovenous malformations of the lateral ventricle. J Neurosurg. 1985. 63: 185-92
40. Wakai S, Kumakura N, Nagai M. Lobar intracerebral hemorrhage. A clinical radiographic and pathological study of 29 consecutive operated cases with negative angiography. J Neurosurg. 1992. 76: 231-8
41. Wong J, Slomovic A, Ibrahim G, Radovanovic I, Tymianski M. Microsurgery for ARUBA trial (a randomized trial of unruptured brain arteriovenous malformation)-eligible unruptured brain arteriovenous malformations. Stroke. 2017. 48: 136-44
42. Yang W, Caplan JM, Ye X, Wang JY, Braileanu M, Rigamonti D. Racial associations with hemorrhagic presentation in cerebral arteriovenous malformations. World Neurosurg. 2015. 84: 461-9