- Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
Correspondence Address:
Ha Son Nguyen
Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, USA
DOI:10.4103/2152-7806.172535
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nguyen HS, Doan N, Wolfla C, Pollock G. Fenestration of bone flap during interval autologous cranioplasty. Surg Neurol Int 24-Dec-2015;6:190
How to cite this URL: Nguyen HS, Doan N, Wolfla C, Pollock G. Fenestration of bone flap during interval autologous cranioplasty. Surg Neurol Int 24-Dec-2015;6:190. Available from: http://surgicalneurologyint.com/surgicalint_articles/fenestration-of-bone-flap-during-interval-autologous-cranioplasty/
Abstract
Background:Symptomatic extra-axial fluid may complicate cranioplasty and require urgent evacuation. Fenestration (F) of the bone flap may encourage extra-axial fluid absorption; however, literature has not explored this technique.
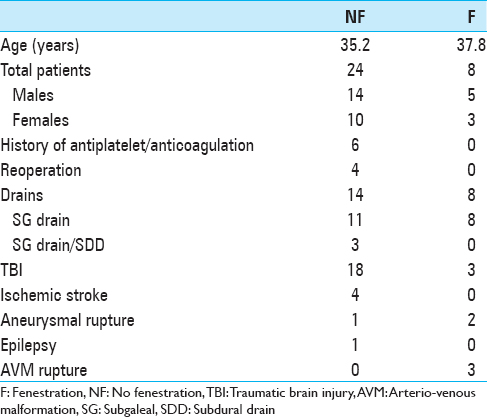
Methods:Thirty-two consecutive patients who underwent interval autologous cranioplasty were divided into two groups: Fenestration, n = 24, and no fenestration (NF), n = 8. Fenestration involves placement of twist-drill holes 1–2 cm apart throughout the bone flap. Clinical data (age, sex, underlying pathology for cranioplasty, history of antiplatelet/anticoagulation [A/A], presence of drains, and length of Intensive Care Unit [ICU] stay) were collected. Postoperative volume and midline shift (MLS) were measured. Univariate analysis was performed for continuous variables; Fisher's exact test was performed for categorical variables.
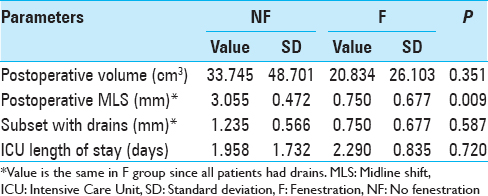
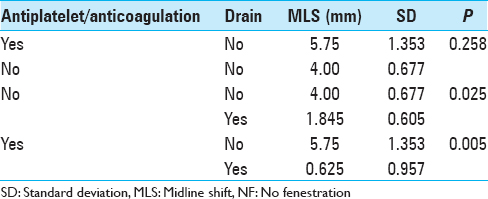
Results:For postoperative volume, NF group exhibited 33.745 ± 48.701 cm3; F group exhibited 20.832 ± 26.103 cm3 (P = 0.351). For MLS, NF group exhibited 3.055 ± 0.472 mm; F group exhibited 0.75 ± 0.677 mm (P = 0.009). MLS for the NF group subset with drains was 1.235 ± 0.566 mm, (P = 0.587 when compared to F group). For ICU length of stay, NF group exhibited 1.958 ± 1.732 days; F group exhibited 2.290 ± 0.835 days (P = 0.720). In NF group, for patients with no A/A, no drain exhibited MLS 4.00 ± 0.677 mm while a drain exhibited 1.845 ± 0.605 mm (P = 0.025); with A/A, no drain exhibited 5.75 ± 1.353 mm while a drain exhibited 0.625 ± 0.957 (P = 0.005). Four NF patients required reoperation compared to zero F patients (P = 0.550).
Conclusion:Presumably, fenestrations augment surface area for extra-axial fluid absorption through the bone flap. Our results, regarding MLS and postoperative volume, provide support for this concept. Accordingly, bone flap fenestration has the potential to reduce extra-axial fluid accumulation.
Keywords: Autologous cranioplasty, bone flap, fenestration
INTRODUCTION
Cranioplasty involves repair of a cranial defect. Although the procedure is relatively straightforward, it has a high complication rate, including risks for infection, bone graft resorption, seizure, and extra-axial fluid accumulation.[
METHODS
The approval of the Institutional Board Review at our institution was obtained prior to the study. Between fall 2012 and spring 2015, 32 patients underwent interval autologous cranioplasty at our institution. Eight neurosurgeons performed the surgeries. The patients were divided into two groups fenestration (F) and no fenestration (NF). Fenestration involves placement of twist-drill holes 1–2 cm apart throughout the bone flap [
Data were entered into SPSS version 22 (IBM Corporation, Armonk, NY, USA) for analysis. Results were expressed as means ± standard error for all descriptive continuous variables. Univariate analysis was performed for continuous variables. Analysis of categorical variables was performed using Fisher's exact test. A P < 0.05 was considered statistically significant.
RESULTS
Tables
DISCUSSION
The overall complication rates for cranioplasty range between 16% and 34% based on various studies.[
Details regarding surgical technique for cranioplasty have been generalized through literature. To our knowledge, the modification for fenestration of the autologous bone flap has not been reported. Several synthetic implants possess fenestration through the implant, including polyether ether ketone implants and titanium mesh; however, limited data exists regarding reoperation rates due to extra-axial fluid accumulation.[
The impact of drain placement during cranioplasty has not been heavily emphasized in literature. There is a technical risk, as drains can be caught in superficial sutures, which require an additional procedure for removal.[
Prior large series did not explore the impact of antiplatelet therapy/anticoagulation on the accumulation of postoperative extra-axial fluid after cranioplasty. In our study, no obvious relationship can be studied with bone flap fenestration, as no patients in the F group had a history of A/A therapy. However, the NF group had 6 patients with that history. In NF group, univariate analysis revealed that with no history of A/A, no drain exhibited a statistically significant larger MLS compared to those with a drain. In addition, univariate analysis revealed that with a history of A/A use, a similar relationship existed regarding the presence of a drain. For NF patients without a drain, presence of antiplatelet therapy/anticoagulation exhibited a larger MLS without reaching statistical significance. An observation of higher MLS is reasonable since antiplatelet therapy/anticoagulation has been linked to higher bleeding risks in prior studies.[
Studies by Gooch et al.[
The limitations of this study include the retrospective analysis, entailing possible selection bias, and loss of patient data. We did not have stratification related to hypertension, tobacco history, or diabetes. We did not study the timing of interval cranioplasty, use of dural adhesion preventive material, or size of the autologous bone flap. Primary pathology for initial craniectomy also varied among patients. Moreover, operative techniques were varied among surgeons; overall, the common practices to avoid fluid accumulation included meticulous hemostasis and avoidance of dural penetration. It is unclear, based on prior literature,[
CONCLUSION
Details regarding surgical technique for cranioplasty have been generalized through literature. To our knowledge, the modification for fenestration of the autologous bone flap has not been reported. Presumably, the fenestrations provide more surface area for drainage of extra-axial fluid through the bone flap. More importantly, potential complications due to the negative pressure and drain placement can be minimized, as the fenestrated bone flap acting as a grid allowing fluid to flow through and removed by the drain placed in the SG space. This technique avoids the drain from applying direct focal negative pressure on the brain surface or cortical vessels. Our results demonstrated a trend for lower postoperative extra-axial fluid accumulation, as well as a statistically smaller MLS, for patients with bone flap fenestration, as opposed to those without fenestration. Moreover, drain placement, after accounting for the history of A/A use, appears to correlate with smaller MLS. Accordingly, we believe bone flap fenestration has the potential to reduce extra-axial fluid accumulation. Similar to ventriculo-SG shunts, fluid absorption may also persist after drains removed due to the continuous shunting of extra-axial fluid through the fenestrated bone flap into SG space, where fluid absorption can occur. Randomized control studies should evaluate this technique as a modification to cranioplasty procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Broughton E, Pobereskin L, Whitfield PC. Seven years of cranioplasty in a regional neurosurgical centre. Br J Neurosurg. 2014. 28: 34-9
2. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
3. Chun HJ, Yi HJ. Efficacy and safety of early cranioplasty, at least within 1 month. J Craniofac Surg. 2011. 22: 203-7
4. Connolly BJ, Pearce LA, Hart RG. Vitamin K antagonists and risk of subdural hematoma: Meta-analysis of randomized clinical trials. Stroke. 2014. 45: 1672-8
5. Flaherty ML. Anticoagulant-associated intracerebral hemorrhage. Semin Neurol. 2010. 30: 565-72
6. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9-
7. Jaben EA, Mulay SB, Stubbs JR. Reversing the effects of antiplatelet agents in the setting of intracranial hemorrhage: A look at the literature. J Intensive Care Med. 2015. 30: 3-7
8. Jaberi J, Gambrell K, Tiwana P, Madden C, Finn R. Long-term clinical outcome analysis of poly-methyl-methacrylate cranioplasty for large skull defects. J Oral Maxillofac Surg. 2013. 71: e81-8
9. Kim SP, Kang DS, Cheong JH, Kim JH, Song KY, Kong MH. Clinical analysis of epidural fluid collection as a complication after cranioplasty. J Korean Neurosurg Soc. 2014. 56: 410-8
10. Klinger DR, Madden C, Beshay J, White J, Gambrell K, Rickert K. Autologous and acrylic cranioplasty: A review of 10 years and 258 cases. World Neurosurg. 2014. 82: e525-30
11. Lee JW, Kim JH, Kang HI, Moon BG, Lee SJ, Kim JS. Epidural fluid collection after cranioplasty: Fate and predictive factors. J Korean Neurosurg Soc. 2011. 50: 231-4
12. Lee L, Ker J, Quah BL, Chou N, Choy D, Yeo TT. A retrospective analysis and review of an institution's experience with the complications of cranioplasty. Br J Neurosurg. 2013. 27: 629-35
13. Martin KD, Franz B, Kirsch M, Polanski W, von der Hagen M, Schackert G. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir (Wien). 2014. 156: 813-24
14. Nagy A, Bognar L, Pataki I, Barta Z, Novak L. Ventriculosubgaleal shunt in the treatment of posthemorrhagic and postinfectious hydrocephalus of premature infants. Childs Nerv Syst. 2013. 29: 413-8
15. Ng ZY, Ang WJ, Nawaz I. Computer-designed polyetheretherketone implants versus titanium mesh (± acrylic cement) in alloplastic cranioplasty: A retrospective single-surgeon, single-center study. J Craniofac Surg. 2014. 25: e185-9
16. Piitulainen JM, Kauko T, Aitasalo KM, Vuorinen V, Vallittu PK, Posti JP. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg. 2015. 83: 708-14
17. Rammos CK, Cayci C, Castro-Garcia JA, Feiz-Erfan I, Lettieri SC. Patient-specific polyetheretherketone implants for repair of craniofacial defects. J Craniofac Surg. 2015. 26: 631-3
18. Sambasivan M. An overview of chronic subdural hematoma: Experience with 2300 cases. Surg Neurol. 1997. 47: 418-22
19. Sucu HK, Gokmen M, Gelal F. The value of XYZ/2 technique compared with computer-assisted volumetric analysis to estimate the volume of chronic subdural hematoma. Stroke. 2005. 36: 998-1000
20. Thien A, King NK, Ang BT, Wang E, Ng I. Comparison of polyetheretherketone and titanium cranioplasty after decompressive craniectomy. World Neurosurg. 2015. 83: 176-80
21. Walcott BP, Kwon CS, Sheth SA, Fehnel CR, Koffie RM, Asaad WF. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013. 118: 757-62
22. Zanaty M, Chalouhi N, Starke RM, Clark SW, Bovenzi CD, Saigh M. Complications following cranioplasty: Incidence and predictors in 348 cases. J Neurosurg. 2015. 123: 182-8