- Center for Neuroscience Service and Research, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150 Kelantan, Malaysia
- Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150 Kelantan, Malaysia
Correspondence Address:
Zamzuri Idris
Center for Neuroscience Service and Research, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150 Kelantan, Malaysia
Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150 Kelantan, Malaysia
DOI:10.4103/2152-7806.143364
Copyright: © 2014 Idris Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Idris Z, Ghazali FH, Abdullah JM. Fibromyalgia and arachnoiditis presented as an acute spinal disorder. Surg Neurol Int 21-Oct-2014;5:151
How to cite this URL: Idris Z, Ghazali FH, Abdullah JM. Fibromyalgia and arachnoiditis presented as an acute spinal disorder. Surg Neurol Int 21-Oct-2014;5:151. Available from: http://surgicalneurologyint.com/4045-autosave-v1/
Abstract
Background:Adhesive arachnoiditis is a chronic, insidious condition that causes debilitating intractable pain and a range of other neurological problems. Its pathophysiology is not well understood. This manuscript discusses its presentations, which can mimic an acute spinal disorder, its hypothetical pathophysiology, treatment, and its relationship with fibromyalgia.
Case Description:The authors present a case of a 47-year-old female who presented with clinical features mimicking an acute spinal disorder but later found to have an adhesive arachnoiditis. She was admitted following a trauma with complaints of back pain and paraplegia. On examination, there was marked tenderness over thoracolumbar spine with lower limbs upper motor neuron weakness. An urgent magnetic resonance imaging (MRI) of the spine revealed multiple lesions at her thoracic and lumbar spinal canals, which did not compress the spinal cord. Therefore, conservative management was initiated. Despite on regular therapies, her back and body pain worsened and little improvement in her limbs power was noted. Laminectomy was pursued and found to have spinal cord arachnoiditis. Subsequently, she was operated by other team members for multiple pelvic masses, which later proved to be benign. After gathering all the clinical information obtained at surgery and after taking detailed history inclusive of cognitive functions, diagnosis of an adhesive arachnoiditis syndrome was made. Currently, she is managed by neuropsychologist and pain specialist.
Conclusion:This case report highlights the importance of knowing an adhesive arachnoiditis syndrome – a rarely discussed pathology by the neurosurgeon, which discloses a significant relationship between immune and nervous systems.
Keywords: Arachnoiditis, autoimmune disease, fibromyalgia, greater limbic system, spinal disorder, spinal trauma
INTRODUCTION
An acute spinal disorder commonly presents as an emergency. Examples of acute spinal disorders are fractured trauma, intraspinal hematomas, and abscess.[
CASE REPORT
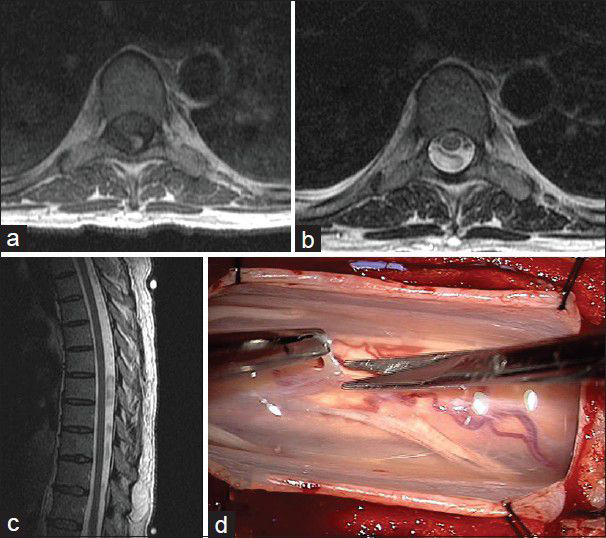
A 47-year-old female with background history of heavy consumption of alcohol at younger age initially presented to us with an acute back pain at the lower thoracic and upper lumbar spines, which was associated with an acute onset of paraparesis with Medical Research Council (MRC) power of 1, paraesthesia and discomfort at voiding following a traumatic event during travelling on a speed boat to an island at a time of heavy waves season. The urgent magnetic resonance imaging (MRI) of the thoracolumbar spine disclosed an abnormal sizeable mixed hypo- and hyperintensed lesions on T1 and T2 [Figure
Laminectomy was completed from T9 to T11 and the intraoperative finding is shown in
Figure 2
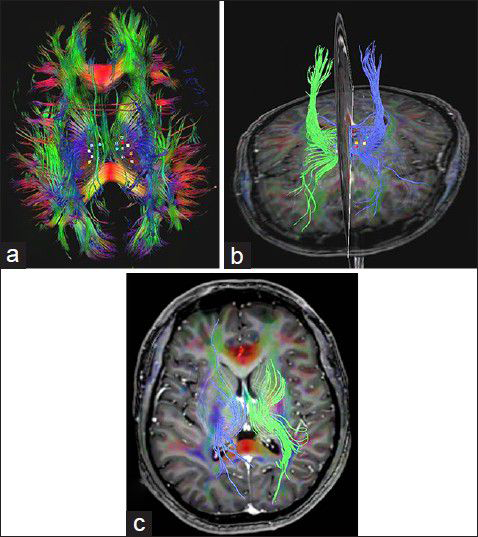
(a and b) Patient's diffusion tensor imaging and tractography disclosed marked increased in fibers density and also fractional anisotropy (FA values) at both thalamus when compared with a healthy subject of nearly similar age (c). The mean FA values for right and left thalamus for the patient were 0.450 and 0.457, whereas for the control were 0.391 and 0.395, respectively
DISCUSSION
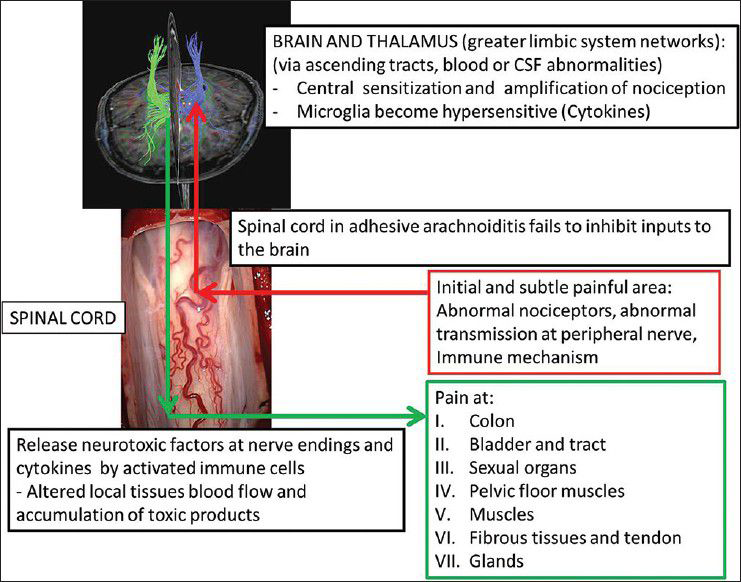
This is a complex and confusing case in such a way that neurosurgeons hardly have much exposure on clinical syndrome called ‘an adhesive arachnoiditis syndrome’. Pain is such a common presentation for this condition, but when it is associated with focal neurological deficits, such as limbs weakness and bladder disturbances, urgent imaging is often indicated. However, musculoskeletal and spinal imagings can be misleading to the clinicians who are ignorant of adhesive arachnoiditis syndrome. In this patient, the spinal neuroimaging (MRI) obviously did not show any compressive effect onto the spinal cord and there was no bony fracture or abnormality, therefore the initial conservative management should have been continued despite slow improvements in muscles power. Lack of knowledge in arachnoiditis syndrome among the clinicians is an obvious reason for her to have multiple surgeries. More diffused and global pain with marked muscles tenderness (misinterpreted as guarding) should have alerted the clinicians toward more chronic pain pathology and neurosurgeon should have been aware and able to correlate the initial histopathological spinal arachnoiditis findings with adhesive arachnoiditis syndrome. Obviously, this case is not a simple case because clinical features of persistent pelvic and abdominal pain and tenderness as well as cystitis-like syndrome with abnormal radiological findings of multiple cystic lesions in the liver and ovaries and abnormal spinal MRIs had also misled the managing teams to proceed with an endoscopic surgery. Unnecessary surgeries though could have been avoided if all the involved clinicians were aware and had sufficient knowledge in this syndrome; in fact, unnecessary spinal surgery in an arachnoiditis patient can cause exacerbation of the condition via activation of already abnormal nociception and peripheral nerve transmission.
Adhesive arachnoiditis syndrome: What is it
The pathophysiology for chronic adhesive arachnoiditis is not completely understood. Arachnoiditis is chronic inflammation of the arachnoid layer of the meninges. Agents that trigger inflammation include direct inoculation such as dye, medications for spinal procedures, blood during the surgery or after subarachnoid hemorrhage, and possibly systemic inoculation via body toxin overload.[
Regarding arachnoiditis in our patient, it seems unlikely that acute trauma (direct inoculation of noxious agent) is causing the arachnoiditis (secondary arachnoiditis). The MRI done within one day of trauma did not favor acute blood clots because of their signals, multiple and inside the arachnoid spaces, which did not cause cord compression. Therefore, theory of resolving acute hematomas that leads to arachnoiditis cannot be entertained. The arachnoiditis seems likely to have been existing long before the history of boat trauma. The etiology for this spontaneous or primary arachnoiditis is unknown. This is possibly related to an autoimmune disorder that links it (via blood) with chronic arachnoiditis (i.e, systemic inoculation-toxin overload). Noteworthy that some patients who suffer from autoimmune disorders such as Sjogren's syndrome, systemic lupus erythematosus do have clinical features associated with this syndrome or fibromyalgia.[
Treatment for adhesive arachnoiditis syndrome and its relationship with fibromyalgia
Managing adhesive arachnoiditis syndrome requires multidisciplinary approach.[
Currently, ‘adhesive arachnoiditis syndrome’ is rarely being discussed among clinicians. This is not because of its rarity but possibly because of ignorance, lack of extensive research for this condition and possibly wrong labeling of the patients suffering from ‘adhesive arachnoiditis syndrome’ with other clinical conditions such as fibromyalgia, failed back surgery syndrome, failed back syndrome, or chronic spinal meningitis. Fibromyalgia is also known as fibrositis. The term fibromyalgia is widely used nowadays and means pain in muscles and fibrous tissues. The pain has specific feature that is global or widespread in nature and often associated with marked muscles or tendon tenderness. Additional sources of pain are abdominal (muscles and colon), pelvic organs (pelvic girdles, urinary, and sexual organs), headache, eye pain, and sore throat. Chronic and widespread pain of muscle origin are reflected in the American College of Rheumatology (ACR) criteria to properly diagnose fibromyalgia.[
CONCLUSION
Adhesive arachnoiditis syndrome is a chronic disease, which implicates nervous, immune, and visceromusculoskeletal systems as important entities in its pathophysiology. Interestingly, its presentation can mimic an acute spinal disorder. Therefore, treating neurosurgeon should be more aware of this syndrome; and practice taking detailed clinical history, do thorough physical examination, and caution in interpreting results of neuroinvestigations.
The discussion of this case presentation indicates that the patient's symptoms progressed from low back pain with lower extremity neurological symptoms to more widespread pain associated with multiple areas of muscle tenderness. The earlier symptom complex is consistent with the diagnosis, ultimately made, of adhesive arachnoiditis and the latter syndrome is clearly more consistent with the diagnosis of fibromyalgia. The earlier symptoms were limited to the back and lower extremities and not associated with multiple muscular tender points. Her past medical and psychosocial histories are not given, so the origin of an inflammatory condition of the spinal canal, such as prior bacterial, tubercular or fungal infection is not ruled out and a psychological source for some of her symptoms also remains specifically undiagnosed, although she did have a prior history of excess alcohol use, which could play a role. The later syndrome with multiple muscle pain and tenderness is quite consistent with the diagnosis of fibromyalgia in which psychosocial issues have become notably prominent. She came under the care of a neuropsychologist so such issues were likely considered and accounted for during treatment. In such a circumstance past history, such as abuse during childhood often plays a major role and her gender is consistent with the fibromyalgia disorder.
As the authors suggest, a multidisciplinary approach to the patient's condition wherein the various surgeons treating her case regularly conferenced with the neuropsychologist and pain specialist also treating her may have reduced the number of operations she had carried out, and may have impacted on the ultimate clinical outcome of her case.[
Commentary
- Department of Neurosurgery, University of Illinois, Chicago, IL, USA
References
1. Austin JW, Afshar M, Fehlings MG. The relationship between localized subarachnoid inflammation and parenchymal pathophysiology after spinal cord injury. J Neurotrauma. 2012. 29: 1838-49
2. Behm FG, Gavin IM, Karpenko O, Lindgren V, Gaitonde S, Gashkoff PA. Unique immunologic patterns in fibromyalgia. BMC Clin Pathol. 2012. 12: 25-
3. Cavanaugh JM. Neural mechanisms of lumbar pain. Spine (Phila Pa 1976). 20: 1804-9
4. Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clinic Proc. 2011. 86: 907-11
5. Desmeules J, Chabert J, Rebsamen M, Rapiti E, Piguet V, Besson M. Central Pain Sensitization, COMT Val158Met Polymorphism, and Emotional Factors in Fibromyalgia. J Pain. 2014. 15: 129-35
6. Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003. 48: 1420-9
7. Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002. 46: 1333-43
8. Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK. Augmented central pain processing in vulvodynia. J Pain. 2013. 14: 579-89
9. Hayashi K, Nagano J, Hattori S. Adhesive arachnoiditis after percutaneous fibrin glue treatment of a sacral meningeal cyst. J Neurosurg Spine. 2014. 20: 763-6
10. Kochany JZ, Tran ND, Sarria JE. Increasing back and radicular pain 2 years following intrathecal pump implantation with review of arachnoiditis. Pain Med. 2013. 14: 1658-63
11. Koerts G, Rooijakkers H, Abu-Serieh B, Cosnard G, Raftopoulos C. Postoperative spinal adhesive arachnoiditis presenting with hydrocephalus and cauda equina syndrome. Clin Neurol Neurosurg. 2008. 110: 171-5
12. Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: A literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003. 26: 1-49
13. Mitsuyama T, Asamoto S, Kawamata T. Novel surgical management of spinal adhesive arachnoiditis by arachnoid microdissection and ventriculo-subarachnoid shunting. J Clin Neurosci. 2011. 18: 1702-4
14. Nieuwenhuys R, Voogd , Huijzen CV.editors. Greater limbic system. Wurzburg, Berlin: Springer-Verlag, Heidelberg; 2008. p.
15. Nieuwenhuys R, Veening JG, van Domburg P. Core and paracores; some new chemoarchitectural entities in the mammalian neuraxis. Acta Morphol Neerl Scand. 1988. 26: 131-63
16. O’Phelan KH, Bunney EB, Weingart SD, Smith WS. Emergency neurological life support: Spinal cord compression (SCC). Neurocrit Care. 2012. 17: S96-101
17. Petty PG, Hudgson P, Hare WS. Symptomatic lumbar spinal arachnoiditis: Fact or fallacy?. J Clin Neurosci. 2000. 7: 395-9
18. Ramli N, Merican AM, Lim A, Kumar G. Post-traumatic arachnoiditis: An unusual cause of Brown-Sequard syndrome. Eur Radiol. 2001. 11: 2011-4
19. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: A meta-analysis of 915 patients. Neurosurg Rev. 2000. 23: 175-204
20. Rodriguez-Pinto I, Agmon-Levin N, Howard A, Shoenfeld Y. Fibromyalgia and cytokines. Immunol Lett. 2014. p.
21. . Available from: http://www.cofwa.org/AASYNDROME.10.03.pdf .
22. Smith HS, Barkin RL. Fibromyalgia syndrome: A discussion of the syndrome and pharmacotherapy. Am J Ther. 2010. 17: 418-39
23. Stein DM, Roddy V, Marx J, Smith WS, Weingart SD. Emergency neurological life support: Traumatic spine injury. Neurocrit Care. 2012. 17: S102-11
24. Vaughan D, Bolger C, O’Brien DF. An interesting case of primary spinal arachnoiditis. Br J Neurosurg. 2012. 26: 555-7
25. Whetstone KE, Crane DA. Cauda equina syndrome resulting from lumbar arachnoiditis after intracranial subarachnoid hemorrhage: A case report. PM R. 2013. 5: 539-41
26. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010. 62: 600-10
27. Wolfe F, Hauser W. Fibromyalgia diagnosis and diagnostic criteria. Ann Med. 2011. 43: 495-502
28. Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: A pilot study. Pain Med. 2009. 10: 663-72
29. Lempp HK(1), Hatch SL, Carville SF, Choy EH. Patients experiences of living with and receiving treatment for fibromyalgia syndrome: a qualitative study. BMC Musculoskelet Disord. 2009. 10: 124-
30. Petzke F. CNS processing of pain in functional somatic syndromes. Article in German Schmerz. 2010. 24: 146-55