- Neurosurgical Clinic, Azienda Ospedaliera Universitaria Policlinico (AOUP) “Paolo Giaccone”, Post Graduate Residency Program in Neurologic Surgery, Department of Biomedicine Neurosciences and Advanced Diagnostics, School of Medicine, University of Palermo,
- Department of Neuroradiology, Azienda di Rilievo Nazionale ed Alta Specializzazione (ARNAS) Civico Hospital, Palermo,
- Department of Neurosurgery, Fondazione Policlinico Universitario A. Gemelli Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Palermo, Italy.
Correspondence Address:
Lara Brunasso, Neurosurgical Clinic, AOUP “Paolo Giaccone”, Post Graduate Residency Program in Neurologic Surgery, Department of Biomedicine Neurosciences and Advanced Diagnostics, School of Medicine, University of Palermo, Palermo, Italy.
DOI:10.25259/SNI_1113_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lara Brunasso1, Nicola Casamassima2, Sergio Abrignani2, Carmelo Lucio Sturiale3, Francesca Incandela2, Giuseppe Roberto Giammalva1, Domenico Gerardo Iacopino1, Rosario Maugeri1, Giuseppe Craparo2. Flow diversion for indirect carotid-cavernous fistula: Still an off-label indication?. 24-Feb-2023;14:65
How to cite this URL: Lara Brunasso1, Nicola Casamassima2, Sergio Abrignani2, Carmelo Lucio Sturiale3, Francesca Incandela2, Giuseppe Roberto Giammalva1, Domenico Gerardo Iacopino1, Rosario Maugeri1, Giuseppe Craparo2. Flow diversion for indirect carotid-cavernous fistula: Still an off-label indication?. 24-Feb-2023;14:65. Available from: https://surgicalneurologyint.com/surgicalint-articles/12174/
Abstract
Background: Flow diversion (FD) is an established treatment for large or giant wide-necked unruptured intracranial aneurysms. In the past few years, the use of flow diverter devices was extended to several other “off-label” indications, including solitary or adjunctive treatment to coil embolization for direct (Barrow A type) carotid cavernous fistulas (CCFs). The use of liquid embolic agents still represents the first-line treatment for indirect CCFs. Typically, the ipsilateral inferior petrosal sinus or superior ophthalmic vein (SOV) is the preferred transvenous routes to access CCFs. In some cases, vessel tortuosity or different features make the endovascular access challenging, thus requiring different approaches and strategies. The aim of the study is to discuss rational and technical aspect in treating indirect CCFs referring to the most up-to-date literature. An alternative experience-based endovascular strategy with FD is described.
Methods: We report the case of a 54-year-old woman diagnosed with indirect CCF and treated with flow diverter stent.
Results: After multiple unsuccessful attempts at transarterial right SOV catheterization, a right indirect CCF fed by a single trunk at the ophthalmic origin from the internal carotid artery (ICA) was treated by ICA stand-alone FD. Blood flow was redirect and successfully reduced through the fistula, with immediately postprocedure improvement of the patient’s clinical status (ipsilateral proptosis and chemosis). Ten-months radiological follow-up showed the complete obliteration of the fistula. No adjunctive endovascular treatment was performed.
Conclusion: FD appears a reasonable alternative stand-alone endovascular strategy also for selected difficult-to-access indirect CCFs, when all conventional routes are judged unfeasible. Further investigations will be necessary to better define and support this potential lesson-learned application.
Keywords: Barrow B type fistulas, Carotid cavernous fistula, Endovascular treatment, Flow diversion, Indirect carotid cavernous fistula (Indirect CCF)
INTRODUCTION
Flow-diversion is rapidly demonstrating its versatility in neurovascular procedures and its indication is progressively extending along with their technological advancements, sometimes outpacing the availability of reliable clinical evidence. The implant of flow diverter devices (FDD) was approved for treating unruptured intracranial aneurysms in different topography.[
CASE DESCRIPTION
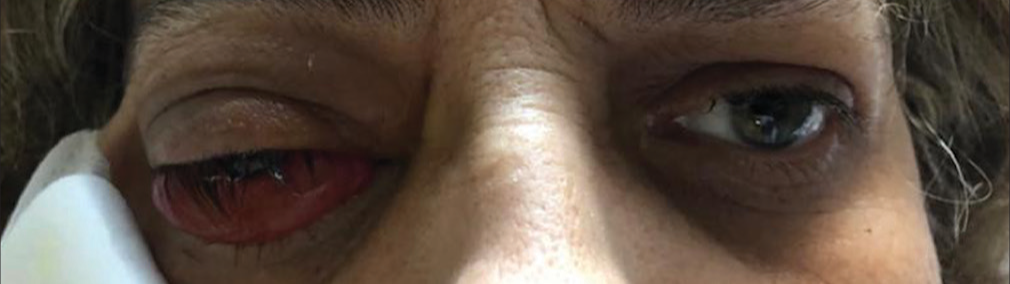
We report the case of a 54-year-old woman complaining progressively right proptosis, chemosis, conjunctival hyperemia, and intense eye pain [
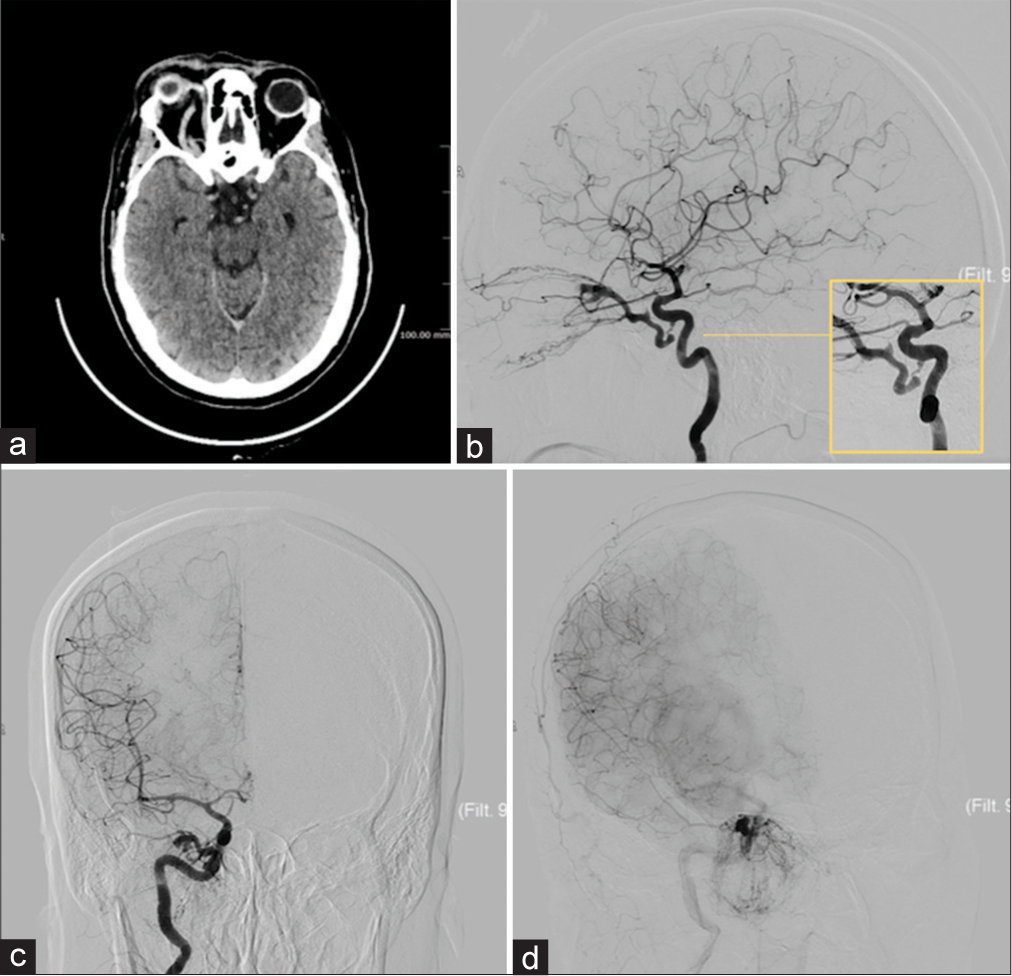
Figure 2:
In (a) preoperative brain computed tomography scan shows significantly dilated right superior ophthalmic vein (SOV) and right exophthalmos, compared to the left side. Angiographic captures (lateral projection in [b], posterior-anterior projection in [c], and oblique projection in (d) show a right indirect carotid cavernous fistula (Barrow type “B”). In the yellow box in (b) the enlargement of the fistula documents the fistulous site filled by a single arterial trunk coming from the inferolateral part of the intracavernous right internal carotid artery and draining into the ipsilateral SOV, conditioning clinical congestion of the right eye, and multiple congested facial and maxillary veins. It should be noted intense SOV opacification during arterial pass of iodate contrast medium.
Description of the endovascular procedure
The first goal of the treatment was to reach the fistulous site through a transarterial route to occlude its distal (venous) part with coils delivery. Under general anesthesia, a selective cerebral digital subtraction angiography (DSA) was performed through a retrograde right transfemoral arterial access, catheterization of the right ICA, and microcatheterization of the right SOV through the fistulous site (long introducer 6F × 80 cm, guide catheter 5.6F × 132 cm, microcatheter 1,7F × 150 cm, microguides 0.014” × 205 cm, 0.08” × 220 cm). Several attempts of a selective microcatheterization of the SOV failed in multiple sessions. Because of the tortuosity of the fistulous feeder and distal stenosis of the fistulous site, it was no possible to introduce the microcatheter to reach the venous compartment. Then, a transvenous route through the ipsilateral inferior petrosal sinus (IPS) was considered, but the irregular appearance and partial thrombosis of the right IPS made no possible a venous catheterization. On the other and, a direct cannulation of the ipsilateral SOV was considered as a major invasive procedure with potential severe complications especially for oculomotor nerve function. After a second multidisciplinary board discussion, the alternative choice was to place a flow diverter stent to cover the segment of the ICA where the fistulous site arises. The rationale for an off-label flow diverter positioning is that flow forces may change becoming turbulent through the fistula, thus favoring a progressive process of thrombosis. Before the stent placement, a manual occlusion test of the left common carotid artery revealed a good compensation with no significant hemodynamic alterations in the anterior circulation. Under general anesthesia through retrograde right transfemoral arterial access, and selected microcatheterization of the right ICA (long introducer 6F × 80 cm, guide catheter 5.6F × 132 cm, microcatheter 2,9F × 150 cm, microguides 0.014” × 205 cm), the fistulous site was reaching. The flow-diverter stent (Derivo©) was correctly opened demonstrating a subsequent angiographic elimination of the CCF with preserved patency of ICA and ophthalmic artery [
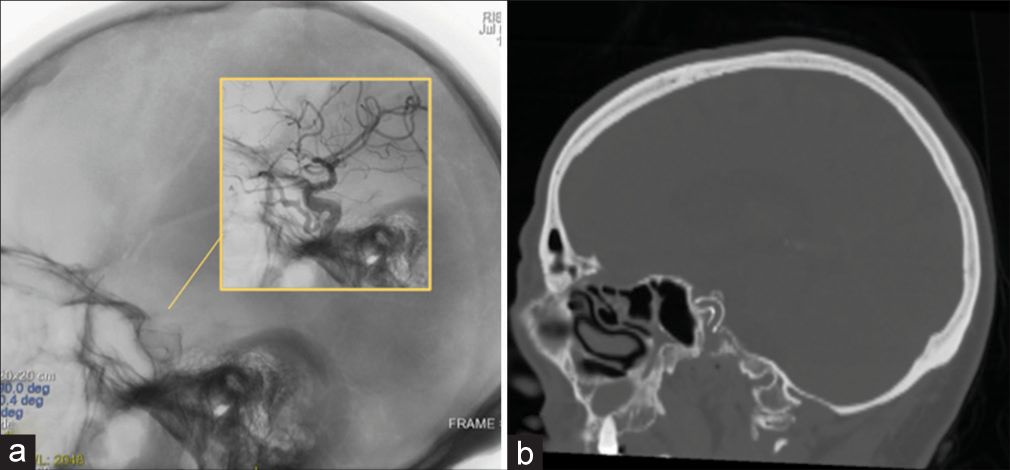
Figure 3:
Intraoperative angiographic capture in lateral projection (a) shows the correct flow-diverter stent placement (Derivo©) within the right internal carotid artery (ICA), and subsequently angiographic elimination of the fistulous link and preserved patency of the vessels (ICA, ophthalmic artery) without complications (enlarged in the yellow box). In (b) sagittal bone-window head computed tomography scan a view of the implanted stent.
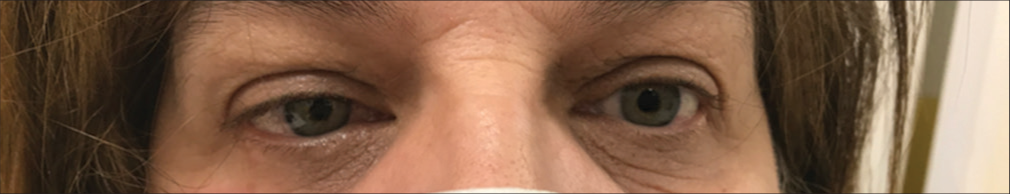
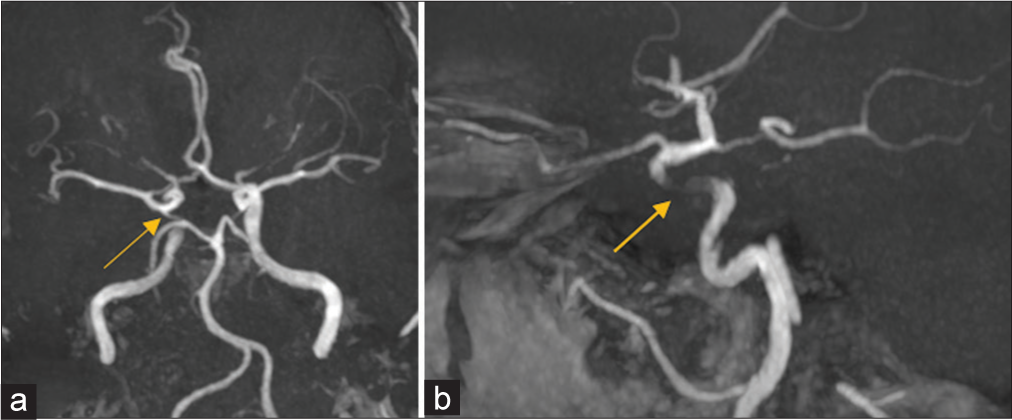
Figure 5:
Intraoperative angiographic capture in lateral projection 10-months magnetic resonance angiography follow-up in (a and b) show normal preserved patency of the Circle of Willis’ vessels, the lack of signal in the right ICA (yellow arrow in a and b) as an indirect sign of the flow-diverter stent placement, and the fistula line closed and entirely excluded.
DISCUSSION
The use of flow diverter stents is rapidly spreading as safe and effective treatment for intracranial aneurysms with different morphology and topography, showing a high rate of aneurysms obliteration over time and a reasonably low incidence of complications.[
CONCLUSION
Although further studies are needed to verify its effectiveness and safety, nowadays, a possible role of flow diverters implant should be taken in mind as a last choice also for indirect Barrow B type CC fistulas.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alaraj A, Kim B, Oh G, Aletich V. Blind endovascular catheterization and direct access of an occluded superior ophthalmic vein for treatment of carotid cavernous fistula. BMJ Case Rep. 2013. 12: bcr2013010704
2. Amuluru K, Al-Mufti F, Gandhi CD, Prestigiacomo CJ, Singh IP. Direct carotid-cavernous fistula: A complication of, and treatment with, flow diversion. Interv Neuroradiol. 2016. 22: 569-76
3. Benndorf G, Bender A, Lehmann R, Lanksch W. Transvenous occlusion of dural cavernous sinus fistulas through the thrombosed inferior petrosal sinus: Report of four cases and review of the literature. Surg Neurol. 2000. 54: 42-54
4. Castaño C, Remollo S, García-Sort R, Domínguez C, Terceño M. Treatment of Barrow type ‘B’ carotid cavernous fistulas with flow diverter stent (Pipeline). Neuroradiol J. 2017. 30: 607-14
5. Dmytriw AA, Phan K, Moore JM, Pereira VM, Krings T, Thomas AJ. On flow diversion: The changing landscape of intracerebral aneurysm management. AJNR Am J Neuroradiol. 2019. 40: 591-600
6. Ellis JA, Goldstein H, Connolly ES, Meyers PM. Carotid-cavernous fistulas. Neurosurg Focus. 2012. 32: E9
7. Gemmete JJ, Ansari SA, Gandhi DM. Endovascular techniques for treatment of carotid-cavernous fistula. J Neuro Ophthalmol. 2009. 29: 62-71
8. Gemmete JJ, Chaudhary N, Pandey A, Ansari S. Treatment of carotid cavernous fistulas. Curr Treat Options Neurol. 2010. 12: 43-53
9. Higashida RT, Hieshima GB, Halbach VV, Bentson JR, Goto K. Closure of carotid cavernous sinus fistulae by external compression of the carotid artery and jugular vein. Acta Radiol Suppl. 1986. 369: 580-3
10. Lekkhong E, Pongpech S, Ter Brugge KG, Jiarakongmun P, Willinsky R, Geibprasert S. Arteriovenous shunts through occluded venous segments : Experience in 51 patients. AJNR Am J Neuroradiol. 2011. 32: 1738-44
11. Limbucci N, Leone G, Renieri L, Nappini S, Cagnazzo F, Laiso A. Expanding indications for flow diverters: Distal aneurysms, bifurcation aneurysms, small aneurysms, previously coiled aneurysms and clipped aneurysms, and carotid cavernous fistulas. Neurosurgery. 2020. 86: S85-94
12. Nossek E, Zumofen D, Nelson E, Raz E, Potts MB, Desousa KG. Use of Pipeline embolization devices for treatment of a direct carotid-cavernous fistula. Acta Neurochir (Wien). 2015. 157: 1125-9 discussion 1130
13. Patel PD, Chalouhi N, Atallah E, Tjoumakaris S, Hasan D, Zarzour H. Off-label uses of the Pipeline embolization device: A review of the literature. Neurosurg Focus. 2017. 42: E4
14. Pradeep N, Nottingham R, Kam A, Gandhi D, Razack N. Treatment of post-traumatic carotid-cavernous fistulas using pipeline embolization device assistance. BMJ Case Rep. 2015. 2015: bcr2015011786
15. Quäschling U, Kläver M, Richter C, Hamerla G, Mucha S, Scherlach C. Flow diversion in challenging vascular anatomies: The use of low profile stent retrievers for safe and accurate positioning of the microcatheter. CVIR Endovasc. 2020. 3: 19
16. Reis CV, Gonzalez FL, Zabramski JM, Hassan A, Deshmukh P, Albuquerque FC. Anatomy of the superior ophthalmic vein approach for direct endovascular access to vascular lesions of the orbit and cavernous sinus. Neurosurgery. 2009. 64: 318-23
17. Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: Anatomy, classification, and treatment. Neurosurg Clin N Am. 2005. 16: 279-95
18. Stamatopoulos T, Anagnostou E, Plakas S, Papachristou K, Lagos P, Samelis A. Treatment of carotid cavernous sinus fistulas with flow diverters. A case report and systematic review. Interv Neuroradiol. 2022. 28: 70-83
19. Sur S, Menaker SA, Alvarez C, Chen S, Shah SS, Peterson EC. Multimodal management of carotid-cavernous fistulas. World Neurosurg. 2020. 133: e796-803
20. Wendl CM, Henkes H, Moreno RM, Ganslandt O, Bäzner H, Pérez MA. Direct carotid cavernous sinus fistulae: Vessel reconstruction using flow-diverting implants. Clin Neuroradiol. 2017. 27: 493-501
21. Yamashita K, Taki W, Nishi S, Sadato A, Nakahara I, Kikuchi H. Transvenous embolization of dural caroticocavernous fistulae: Technical considerations. Neuroradiology. 1993. 35: 475-9
22. Yüce G, Doğan A. A case of indirect carotid cavernous fistula presenting with proptosis and pulsatile exophthalmos. Gulhane Med J. 2021. 63: 160-2