- Department of Neurosurgery, McMaster University, Hamilton, Ontario, Canada.
- Department of Health Sciences Library, McMaster University, Hamilton, Ontario, Canada.
Correspondence Address:
Mohamad Ali Kesserwan
Department of Neurosurgery, McMaster University, Hamilton, Ontario, Canada.
DOI:10.25259/SNI_824_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohamad Ali Kesserwan1, Husain Shakil1, Melissa Lannon1, Ryan McGinn1, Laura Banfield2, Siddharth Nath1, Mazen Alotaibi1, Ekkehard Kasper1, Sunjay Sharma1. Frame-based versus frameless stereotactic brain biopsies: A systematic review and meta-analysis. 10-Feb-2021;12:52
How to cite this URL: Mohamad Ali Kesserwan1, Husain Shakil1, Melissa Lannon1, Ryan McGinn1, Laura Banfield2, Siddharth Nath1, Mazen Alotaibi1, Ekkehard Kasper1, Sunjay Sharma1. Frame-based versus frameless stereotactic brain biopsies: A systematic review and meta-analysis. 10-Feb-2021;12:52. Available from: https://surgicalneurologyint.com/surgicalint-articles/10576/
Abstract
Background: Stereotactic brain biopsy techniques have been a focus of rapid technological innovation. The recent advent of frameless stereotaxy has invited the question of whether it can provide the same diagnostic yield as frame-based techniques, without increasing risk of harm to patients. The goal of this meta-analysis was to compare each of these techniques in terms of yield and safety.
Methods: We independently searched four databases for English studies comparing frameless and frame-based stereotactic brain biopsies. Our primary outcome was biopsy diagnostic yield. Our secondary outcomes included mortality, morbidity (e.g., symptomatic postbiopsy intracranial hemorrhage, asymptomatic postbiopsy intracranial hemorrhage, new postbiopsy neurological deficit, and postbiopsy seizure), and frequency of repeat biopsy. We calculated pooled estimates and relative risks for dichotomous outcomes using Review Manager 5.3, with corresponding 95% confidence intervals.
Results: A total of 3256 stereotactic brain biopsies (2050 frame based and 1206 frameless), from 20 studies, were included in our final analysis. The results did not demonstrate any significant difference between the two stereotactic systems in terms of diagnostic yield (risk ratio [RR] 1.00, 95% confidence interval [CI] 0.99–1.02, P = 0.64, I2 = 0%). The only significant difference was the increased frequency of asymptomatic hemorrhages in the frameless group (RR 1.37, 95% CI 1.06–1.75, P = 0.01, I2 = 0%). Application of Grading of Recommendations Assessment, Development, and Evaluation to the results yielded very low quality of all outcomes.
Conclusion: Based on very low-quality evidence, both frame-based and frameless stereotaxy are safe and effective for biopsy of intracranial tumors. Further study of patient preference and cost comparing analysis is required to identify if either modality should be preferred.
Keywords: Brain biopsy, Frame-based brain biopsy, Frameless brain biopsy, Stereotactic biopsy, Stereotactic brain biopsy, Stereotaxy
INTRODUCTION
Brain biopsy is a surgical procedure whereby a small amount of intracranial tissue is obtained for pathological analysis. It is conducted for diagnostic purposes on which treatment recommendations are based. While the basic procedural principles (e.g., need of imaging for target visualization and definition of spatial reference points for targeting) have remained largely unchanged, the specific methods of obtaining samples of brain tissue have evolved overtime from freehand craniotomy procedures into minimally invasive stereotactic techniques. Modern approaches, aided by further evolving medical imaging modalities, allow for the sampling of very small and even deep-seated intracranial lesions with safety, accuracy, precision, and reliability.[
In general terms, stereotactic techniques allow for the brain to be mapped onto a three-dimensional (3D) coordinate system by utilizing preoperative imaging, typically magnetic resonance imaging (MRI) or computed tomography (CT), in conjunction with a radiopaque fiducial set or fixed frame. Suitable target coordinates are then selected based on the reference system to guide a biopsy needle through a small burr hole toward the intended target point.[
Frame-based techniques have been considered the gold standard for many years, due to their superiority over freehand biopsy.[ Frameless neuronavigation systems utilize a pointer system, a digitizer, a work station, and fiducials. The fiducials are placed on the scalp for preoperative imaging and then used as reference to allow for the definition and calibration of the surgical space relative to the patient’s head on these images. The digitizer registers and then transfers this spatial information to a workstation, which allows a registered pointer and the biopsy probe to be projected onto the preoperative images. This provides accurate intraoperative navigation and targeting of lesions using the preoperative images.[
Conventionally, rigid frame-based biopsy is considered to convey greater precision when compared to frameless options, particularly when targeting small deep-seated lesions. This is due to the hypothesis that frameless techniques require more complex hand-eye coordination and, therefore, may be more prone to drift and tremor.[
The primary outcome for this meta-analysis is biopsy diagnostic yield, with a secondary focus on associated complications, with outcomes including mortality, postbiopsy intracranial hemorrhage, new postbiopsy neurologic deficit, postbiopsy seizure, and frequency of a repeat biopsy.
MATERIALS AND METHODS
Search strategy and study selection
This systematic review and meta-analysis are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis[
Data collection
Data regarding patient and study characteristics, details of the specific biopsy apparatus and other operative details, lesional characteristics, histological yield, postbiopsy bleeding rates, postbiopsy clinical complications, and length of hospital stay were abstracted. The primary outcome investigated in our study is histopathological yield. Diagnostic yield was defined as the proportion of biopsies performed with either stereotactic technique that yielded a definitive diagnosis. The secondary outcomes included incidence of mortality, symptomatic postbiopsy intracranial hemorrhage, asymptomatic postbiopsy intracranial hemorrhage, neurological deficit, seizure, and frequency of repeat biopsy. Mortality included all-cause mortality events postbiopsy, and not necessarily related to the biopsy. Asymptomatic postbiopsy intracranial hemorrhage was defined as hemorrhage noted on imaging, but without any clinical consequences. The data abstraction table is provided in Online Resource 2.
Data analysis
Histological yield and risks of frame-based versus frameless intracranial brain biopsies were pooled using Review Manager (version 5.3, Cochrane Collaboration) by the inverse variance method and random effects analysis model. The pooled results of the seven dichotomous outcomes were presented as risk ratio (RR) with corresponding 95% confidence interval (CI). The statistical significance was defined as P < 0.05. Quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.[
RESULTS
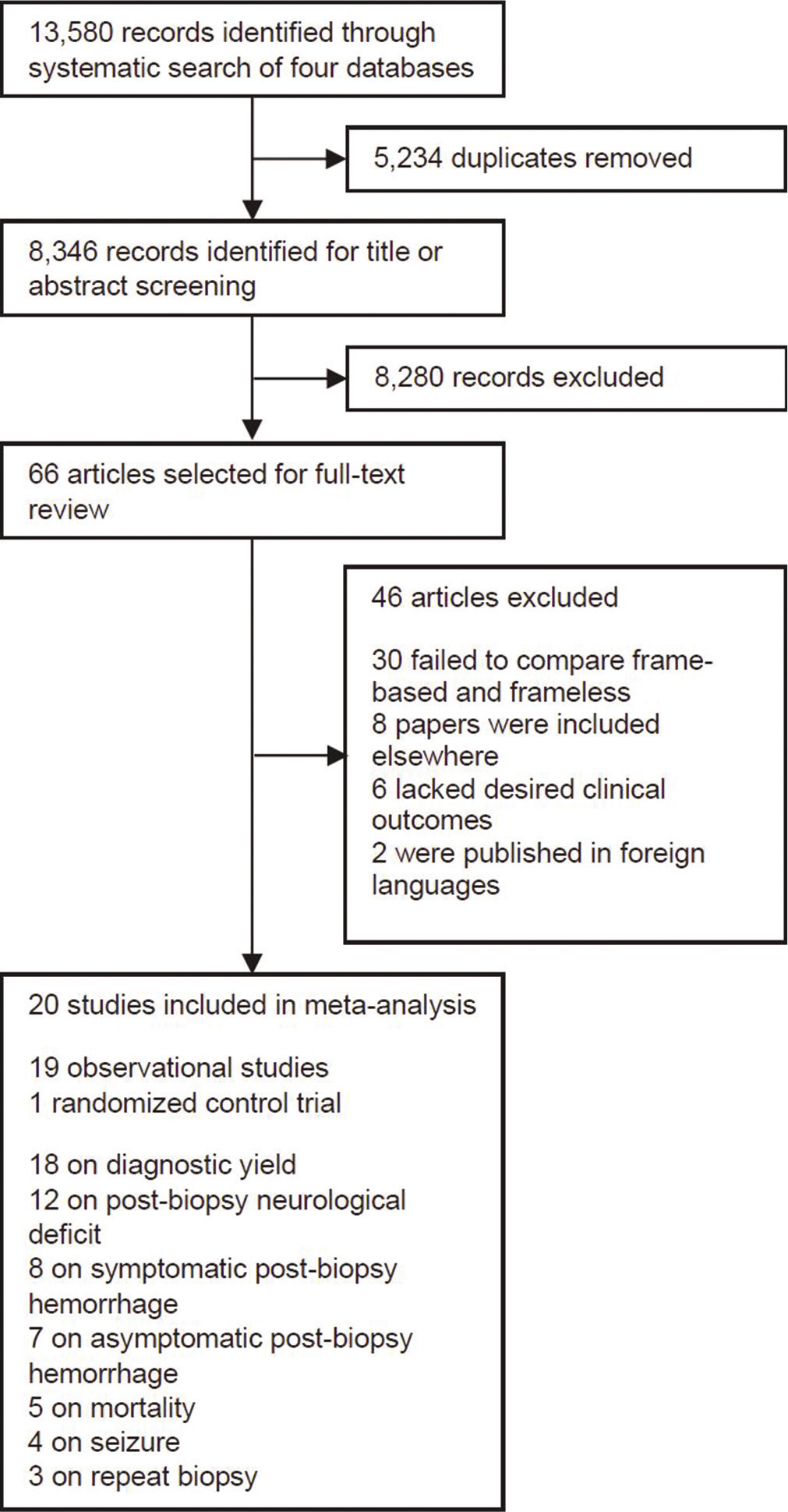
Our search identified 13,580 records with 5234 of these representing duplicate publications and 8280 excluded after screening of title and abstract. In total, 66 full-text articles were assessed in detail for eligibility. Of these remaining articles, 30 were excluded as they did not compare the two stereotactic techniques being assessed with this study, eight papers were repeat publications, six lacked the complete set of desired clinical outcomes for this study, and two were published in languages other than English. In total, 20 studies were included for quantitative synthesis consisting of 19 observational studies and one randomized control study [
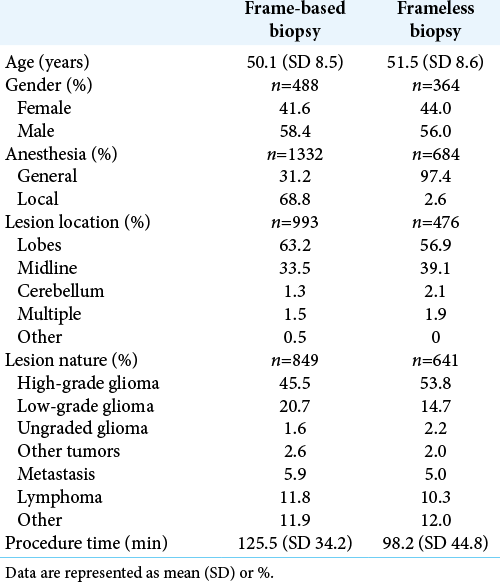
For frame-based brain biopsy, the mean age of participants was 50.1 years (SD 8.5), of whom 41.6% were women. The majority of frame-based biopsies were performed under local anesthesia 68.8%, while only 31.2% employed general anesthesia, with the average total procedure time being 125.5 min (SD 34.2). The nature of the lesions targeted by frame-based biopsies composed of high-grade glioma 45.5%, low-grade glioma 20.7%, ungraded glioma 1.6%, other tumors 2.6%, metastasis 5.9%, lymphoma 11.8%, and other 11.9%. The location of the lesions targeted included lobar 63.2%, midline 33.5%, cerebellum 1.3%, multiple locations 1.5%, and other 0.5% [
For frameless brain biopsy, the mean age of participants was 51.5 years (SD 8.6), of whom 44.0% were women. Almost all biopsies were performed under general anesthesia 97.4%, with only 2.6% of frameless biopsies performed under local anesthesia. The average total procedure time was 98.2 min (SD 44.8). The lesions targeted with the frameless technique included high-grade glioma 53.8%, low-grade glioma 14.7%, ungraded glioma 2.2%, other tumors 2.0%, metastasis 5.0%, lymphoma 10.3%, and other 12.0%. The lesions targeted were lobar 56.9%, midline 39.1%, cerebellar 2.1%, and multiple 1.9% [
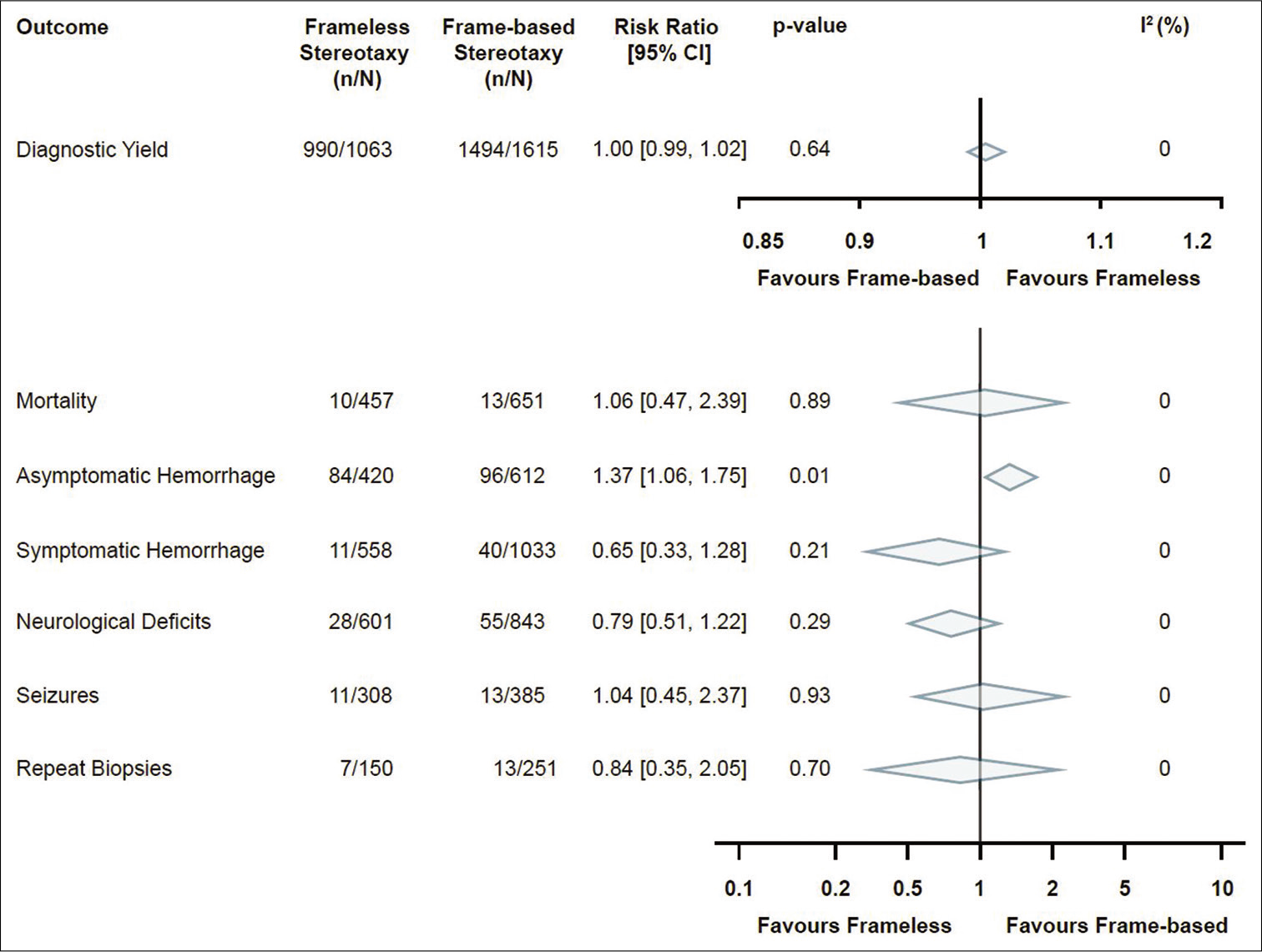
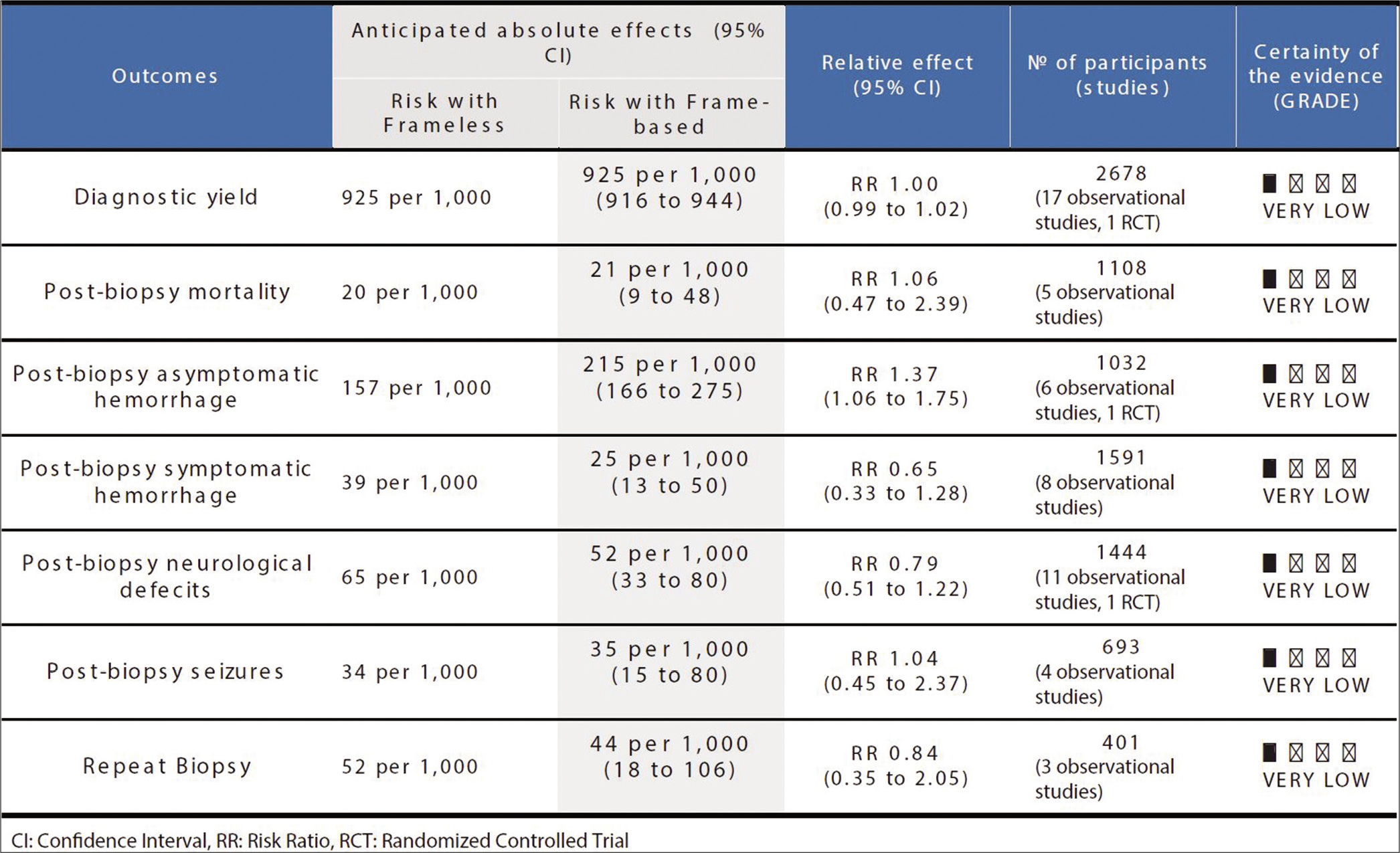
In terms of the primary outcome, diagnostic yield, 18 studies were analyzed with a total of 2678 stereotactic brain biopsies. There were 1063 biopsies in the frameless group (from which diagnostic tissue was obtained in 990 biopsies, 93.1%) and 1615 biopsies in the frame-based group (from which diagnostic tissue was obtained in 1494 biopsies, 92.5%). Ultimately, there was no significant difference between frameless and frame-based diagnostic yield in any of the studies included or the pooled analysis (RR 1.0, 95% CI 0.99–1.02, P = 0.64, I2 = 0%) [
Secondary outcomes included measures of morbidity and mortality. The analysis for postbiopsy mortality included a total of five studies, with 1108 biopsies in total. Of 457 biopsies in the frameless group, there were 10 instances of mortality (2.2%). This compares with 13 cases of mortality in the frame-based group in 651 cases (2.0%). This difference was not found to be significant in either the pooled data (RR 1.06, 95% CI 0.47–2.39, P = 0.89, I2 = 0%) [
The overall quality of available evidence for this meta-analysis was rated as very low using the GRADE approach [
DISCUSSION
This systematic review of literature and subsequent meta-analysis did not demonstrate any significant clinical difference between reported frame-based and frameless stereotactic brain biopsy techniques. Postbiopsy asymptomatic hemorrhage incidence was the only outcome displaying a significant difference between the two groups.
This analysis showed that biopsies performed with frame-based techniques, contrary to general belief, did not have a higher diagnostic yield. Some of the confounding factors that may have biased this result to be nonsignificant when comparing the two systems include the indications of using either technique as well as the size and depth of lesions in relation to the number of samples taken. With the potential bias of neurosurgeons preferentially using the frame-based apparatus for deeper seated lesions,[
Among all publications included in the postbiopsy asymptomatic hemorrhage analysis, the study by Michaud et al.[
In the absence of any difference in terms of histologic diagnostic yield or clinically significant complication rates, it is worthwhile considering factors that do differentiate these techniques. Here, in an effort to improve patient experience and operative efficiency, surgeon and patient experience may influence the decision to favor one method over the other. Frameless techniques carry advantages to patients in terms of comfort. Frame-based approaches require fixation of the frame to the patient’s head, and the patient must wear the rigid apparatus for imaging before being taken to the operating room. Although many studies report frame placement as “well tolerated,” there have been a number of reports in functional neurosurgery that provides evidence that frame fixation is quite uncomfortable for patients[
Limitations of our study
The first limitation of this review pertains to the restriction of our study to the four large existing databases and to studies published in the English language. Second, the level of evidence available on the topic was deemed poor, with reported outcomes of very low-quality evidence per the GRADE method. Nineteen of the 20 articles included in this analysis were observational studies, significantly lowering the quality of evidence. This meta-analysis included significantly more frame-based (n = 2050) than frameless (n = 1206) biopsies, which could be a reflection of a lower comfort level with frameless systems in the reporting institutions. It should also be considered that several articles had differing criteria with respect to what was considered a diagnostic specimen. This could have significantly altered the pooled data. In addition, several confounding variables may have impacted whether a specimen was diagnostic, including underlying pathological entity, size of the specimen retrieved, number of samples acquired per surgery, and number of separate tracts and trajectories acquired. However, given that we included published studies from multiple institutions, these factors cannot be well controlled for, unless one performs a controlled trial with a unified tissue acquisition protocol as well as central pathological review of all specimens. There was also a significant difference in the number of studies included in each outcome analysis, ranging from three for repeat biopsies to 18 for diagnostic yield; further limiting the quality of our data. The fewer number of included studies also allowed for individual articles to have a substantial weight percentage in outcome analysis. This is illustrated by the previous example of how Michaud et al.[
To the best of our knowledge, there is only one previously reported meta-analysis comparing frame-based versus frameless stereotactic biopsy.[
CONCLUSION
This meta-analysis provides evidence of noninferiority for frameless biopsy methods when compared to frame-based brain biopsy techniques. There was no significant difference in either biopsy diagnostic yield or the presence of negative clinical outcomes between techniques. As such, we argue that practical considerations, particularly patient experience, should be taken into consideration as a guiding factor in deciding between these two techniques in cases where technical or procedural challenges do not exclude either one of the methods.
In the absence of any evidence to suggest that frameless techniques are less likely to yield the correct histopathological diagnosis, and based on emerging evidence that these techniques are not more likely to cause harm to patients, we suggest to consider this method in routine practice. Further study with granular outcomes such as efficiency, cost-effectiveness, and patient experience as outcomes may mitigate this consideration and are required to develop a comprehensive recommendation of which method to use. However, our study identifies that surgeons should feel confident in both biopsy yield and patient safety regardless of modality used.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alesch F, Kitz K, Koos W, OStertag C. Diagnostic potential of stereotactic biopsy of midline lesions. Acta Neurochir Suppl (Wien). 1991. 53: 33-6
2. Ben-Haim S, Falowski SM. Evaluation of patient perspectives toward awake, frame-based deep-brain stimulation surgery. World Neurosurg. 2018. 111: e601-7
3. Chen CC, Hsu PW, Wu TW, Lee ST, Chang CN, Wei K. Stereotactic brain biopsy: Single center retrospective analysis of complications. Clin Neurol Neurosurg. 2009. 111: 835-9
4. Dammers R, Haitsma IK, Schouten JW, Kros JM, Avezaat CJJ, Vincent AJ. Safety and efficacy of frameless and frame-based intracranial biopsy techniques. Acta Neurochir (Wien). 2008. 150: 23-9
5. Dhawan S, He Y, Bartek J, Alattar AA, Chen CC. Comparison of frame-based versus frameless intracranial stereotactic biopsy: Systematic review and meta-analysis. World Neurosurg. 2019. 127: 16-e4
6. Georgiopoulos M, Ellul J, Chroni E, Constantoyannis C. Efficacy, safety, and duration of a frameless fiducial-less brain biopsy versus frame-based stereotactic biopsy: A prospective randomized study. J Neurol Surg A Cent Eur Neurosurg. 2018. 79: 31-8
7. Grimm F, Naros G, Gutenberg A, Keric N, Giese A, Gharabaghi A. Blurring the boundaries between frame-based and frameless stereotaxy: Feasibility study for brain biopsies performed with the use of a head-mounted robot. J Neurosurg. 2015. 123: 737-42
8. Grunert P, Darabi K, Espinosa J, Filippi R. Computer-aided navigation in neurosurgery. Neurosurg Rev. 2003. 26: 73-99
9. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008. 336: 924-6
10. Higgins J, Green S.editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Oxford: The Cochrane Collaboration; 2011. p.
11. Lefranc M, Capel C, Pruvot-Occean AS, Fichten A, Desenclos C, Toussaint P. Frameless robotic stereotactic biopsies: A consecutive series of 100 cases. J Neurosurg. 2014. 122: 342-52
12. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ. 2009. 339: b2700
13. McGirt MJ, Woodworth GF, Coon AL, Frazier JM, Amundson E, Garonzik I. Independent predictors of morbidity after image-guided stereotactic brain biopsy: A risk assessment of 270 cases. J Neurosurg. 2005. 102: 897-901
14. McInerney J, Roberts DW. Frameless stereotaxy of the brain. Mt Sinai J Med. 2000. 67: 300-10
15. Michaud K, Cantin L, Cottin S, Dandurand C. Frame-based or frameless stereotactic biopsy: The debate continuous. Neuro Oncol. 2013. 15: iii217-25
16. Mulroy E, Robertson N, Macdonald L, Bok A, Simpson M. Patients’ perioperative experience of awake deep-brain stimulation for parkinson disease. World Neurosurg. 2017. 105: 526-8
17. Nishihara M, Kohmura E, Takeda N, Harada T, Kidoguchi K, Tatsumi S. Diagnostic yield and morbidity by neuronavigation-guided frameless stereotactic biopsy using magnetic resonance imaging and by frame-based computed tomography-guided stereotactic biopsy. Surg Neurol Int. 2014. 5: S421-6
18. Owen CM, Linskey ME. Frame-based stereotaxy in a frameless era: Current capabilities, relative role, and the positive-and negative predictive values of blood through the needle. J Neurooncol. 2009. 93: 139-49
19. Smith JS, Quiñones-Hinojosa A, Barbaro NM, McDermott MW. Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. J Neurooncol. 2005. 73: 173-9
20. Widmann G, Schullian P, Ortler M, Bale R. Frameless stereotactic targeting devices: Technical features, targeting errors and clinical results. Int J Med Robot. 2012. 8: 1-16
21. Woodworth G, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: Comparison of biopsy and open resection specimen. Neurol Res. 2005. 27: 358-62
22. Woodworth GF, McGirt MJ, Samdani A, Garonzik I, Olivi A, Weingart JD. Frameless image-guided stereotactic brain biopsy procedure: Diagnostic yield, surgical morbidity, and comparison with the frame-based technique. J Neurosurg. 2006. 104: 233-7