- Norton Neuroscience Institute, Norton Healthcare, Louisville, Kentucky, USA
- The Brain Tumor Center, Norton Healthcare, Louisville, Kentucky, USA
- Markey Cancer Center, University of Kentucky, Lexington, Kentucky, USA
- The Norton Cancer Institute Radiation Center and Kosair Children's Hospital, Louisville, Kentucky, USA
Correspondence Address:
Aaron C. Spalding
The Brain Tumor Center, Norton Healthcare, Louisville, Kentucky, USA
The Norton Cancer Institute Radiation Center and Kosair Children's Hospital, Louisville, Kentucky, USA
DOI:10.4103/2152-7806.207119
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lisa B. E. Shields, Todd S. Shanks, Andrew J. Shearer, Lauren A. Shelton, Brent J. Shelton, Jonathan Howe, James M. Coons, Brian Plato, Aaron C. Spalding. Frameless image-guided radiosurgery for trigeminal neuralgia. 26-May-2017;8:87
How to cite this URL: Lisa B. E. Shields, Todd S. Shanks, Andrew J. Shearer, Lauren A. Shelton, Brent J. Shelton, Jonathan Howe, James M. Coons, Brian Plato, Aaron C. Spalding. Frameless image-guided radiosurgery for trigeminal neuralgia. 26-May-2017;8:87. Available from: http://surgicalneurologyint.com/surgicalint-articles/frameless-image%e2%80%91guided-radiosurgery-for-trigeminal-neuralgia/
Abstract
Background:Frameless image-guided radiosurgery (IGRS) is a safe and effective noninvasive treatment for trigeminal neuralgia (TN). This study evaluates the use of frameless IGRS to treat patients with refractory TN.
Methods:We reviewed the records of 20 patients diagnosed with TN who underwent frameless IGRS treatments between March 2012 and December 2013. Facial pain was graded using the Barrow Neurological Institute (BNI) scoring system. The initial setup uncertainty from simulation to treatment and the patient intrafraction uncertainty were measured. The median follow-up was 32 months.
Results:All patients’ pain was BNI Grade IV or V before the frameless IGRS treatment. The mean intrafraction shift was 0.43 mm (0.28–0.76 mm), and the maximum intrafraction shift was 0.95 mm (0.53–1.99 mm). At last follow-up, 8 (40%) patients no longer required medications (BNI 1 or 2), 11 (55%) patients were pain free but required medication (BNI 3), and 1 (5%) patient had no pain relief (BNI 5). Patients who did not have prior surgery had a higher odds ratio for pain relief compared to patients who had prior surgery (14.9, P = 0.0408).
Conclusions:Frameless IGRS provides comparable dosimetric and clinical outcomes to frame-based SRS in a noninvasive fashion for patients with medically refractory TN.
Keywords: Frameless, image-guided radiosurgery, radiation, stereotactic radiosurgery, trigeminal neuralgia
INTRODUCTION
Trigeminal neuralgia (TN) causes brief and severe intensity unilateral facial pain that is electric shock-like, shooting, stabbing, or sharp in quality.[
The initial treatment of TN includes medical management with anticonvulsants and other medications, which are typically used for neuropathic pain. Approximately 90% of patients with new-onset TN attain either complete or significant relief of symptoms following medical therapy.[
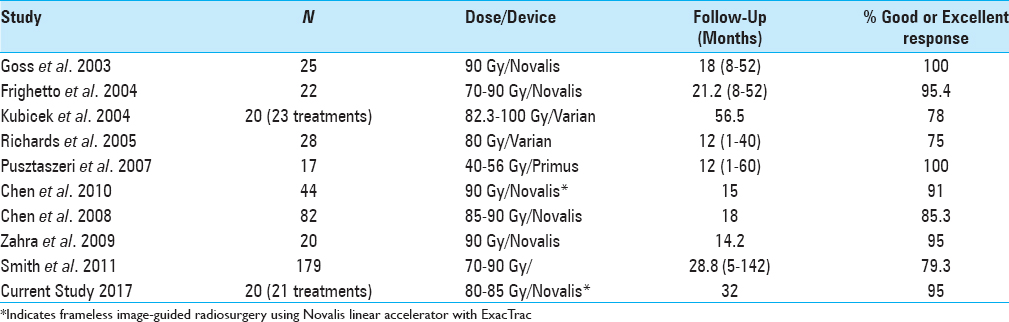
Traditional SRS treatment of TN has used a rigid invasive headframe to deliver between 80–90 Gy to a point within millimeters of the brainstem. We conducted a retrospective study of 20 patients who underwent frameless image-guided radiosurgery (IGRS) by treating a point along the trigeminal nerve ranging from the dorsal root entry (DRE) zone to the retro Gasserian ganglion. This approach ensured submillimeter accuracy and allowed more complicated SRS planning over several days. We present our clinical findings and patient positioning data pertaining to the use of frameless IGRS using the BrainLAB Novalis ExacTrac system (BrainLAB AG, Feldkirchen, Germany) in the treatment of TN. The present study also compares the patient responses following the treatment for TN with frameless IGRS to frame-based linear accelerator (LINAC) SRS based on historical published data.
MATERIAL AND METHODS
Patient population
Between March 2012 and December 2013, a total of 20 individuals with TN underwent frameless IGRS at our institution. The Institutional Review Board approved the retrospective review of these cases. The primary characteristics of these patients are presented in
All 20 patients were evaluated by a neurosurgeon, neurologist, and radiation oncologist and underwent frameless IGRS treatment. One patient underwent frameless IGRS retreatment after failing the first treatment. Each patient was scored pre- and post- frameless IGRS based on the Barrow Neurological Institute (BNI) pain intensity scoring criteria (I: no pain; II: occasional pain, not requiring medication; III: some pain, controlled with medication; IV: some pain, not controlled with medication; V: severe pain/no pain relief).[
Treatment method
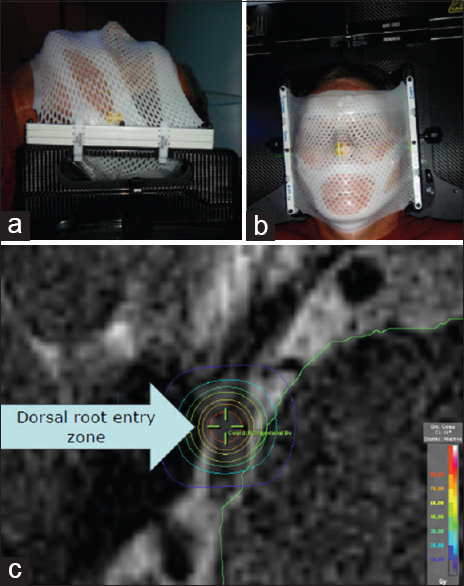
Simulation
After each patient signed the informed consent, a bivalve-style thermoplastic mask was fabricated to immobilize the head. All patients were simulated and treated supine and head-first. Next, a stereotactic localizer frame was attached to the imaging frame, and then patients underwent a 0.6 mm axial slice computed tomography (CT) scan using 40-slice Siemens Sensation Open (Siemens, Munich, Germany) from the vertex to the third cervical vertebra. We obtained a 0.5 mm constructive interference in a steady state (CISS) T2 magnetic resonance imaging (MRI) sequence using a three Tesla magnet with zero gantry tilt and registered the CT and MRI datasets using BrainLAB iPlan RT Image software, versions 4.1.0 and 4.1.1 [
Treatment planning
The affected side trigeminal nerve and organs at risk were contoured by the neurosurgeon and radiation oncologist on the fused dataset. The isocenter was placed in the retrogaussarian space. The SRS plan was prescribed at the isocenter, and all plans used between seven and nine non-coplanar arcs with fixed diameter cones ranging from 4.0 to 7.5 mm aperture, with total scatter factors of 0.669 and 0.815, respectively. Each arc used between 60 and 140 degrees. The mean maximum brainstem dose was 31 Gy (range 8–57 Gy), the mean dose to 0.1 cc of the brainstem was 9 Gy (range 4–15 Gy), and the mean dose to 1 cc of the brainstem was 3 Gy (range 1–6 Gy).
Treatment delivery
Patients were placed in the simulation position on the LINAC treatment couch and the immobilization mask was applied. An optical tracking array for localization was used to determine the initial position. Stereoscopic X-rays were fused with the digitally reconstructed radiographs from simulation, allowing Exactrac to measure the shift from simulation to treatment. Corrections to patient positioning were made for deviations exceeding 0.5 mm and 1° using a robotic couch with six degrees of freedom. The mean setup uncertainty from simulation to treatment was 2.9 mm (±1.5 mm). Before each arc was delivered, an additional set of stereoscopic X-rays was obtained. Total treatment time ranged from 35 to 100 minutes. Three patients received 80 Gy, and 16 received 85 Gy. One patient was scheduled to receive 85 Gy, however, he experienced severe postnasal drip coupled with claustrophobia and was treated with 22.65 Gy.
Quality assurance
There were both LINAC and patient specific quality assurance measures taken to maximize the accuracy of treatment. The cones were commissioned with small-field dosimetry techniques for treatment planning. Immediately prior to treatment delivery, a Winston–Lutz test was performed to verify that the treatment and imaging isocenter were congruent. The resulting root-mean square geometric error was 0.58 ± 0.2 mm.
Follow-up
Patients were assessed 1 month after the procedure and then approximately every 2 to 3 months. Each patient was evaluated by the treating neurosurgeon and/or radiation oncologist. A physical examination with facial sensation was performed, and a BNI score was assigned.
Data analysis
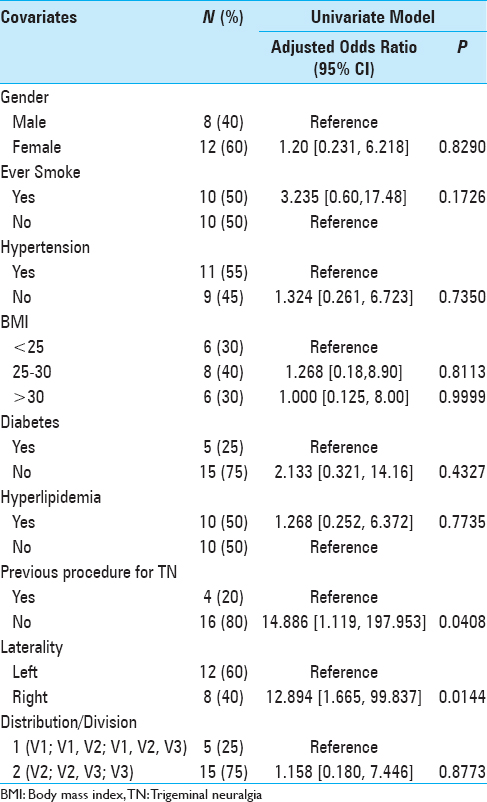
Primary data analyses were focused on assessing the outcome of change in BNI score from study entry to BNI measured at the last follow-up. This score ranged from −1 (change from 4 to a 5 in 1 patient) to a 4 (in 3 patients going from a 5 down to a 1). This score change variable was used to create 3 categories of pain improvement: score change of 3 or 4 = marked improvement (n = 7 patients), score change of 2 = moderate improvement (n = 7 patients), and score change of −1 or 1 = some/none/worse improvement (n = 6). This 3-level ordinal variable was then used as the outcome to assess whether variations in the outcome were due to clinical factors as determined by a proportional odds model using Statistical Analysis Software (SAS) V9.3. The proportional odds assumption was assessed using the score test and was not rejected for any of the covariates examined [
Descriptive analyses for the outcome and relevant covariates are reported as frequencies and percentage [
RESULTS
Between March 2012 and December 2013, a total of 20 patients (12 women and 8 men) diagnosed with TN underwent frameless IGRS [
Intraprocedural positioning
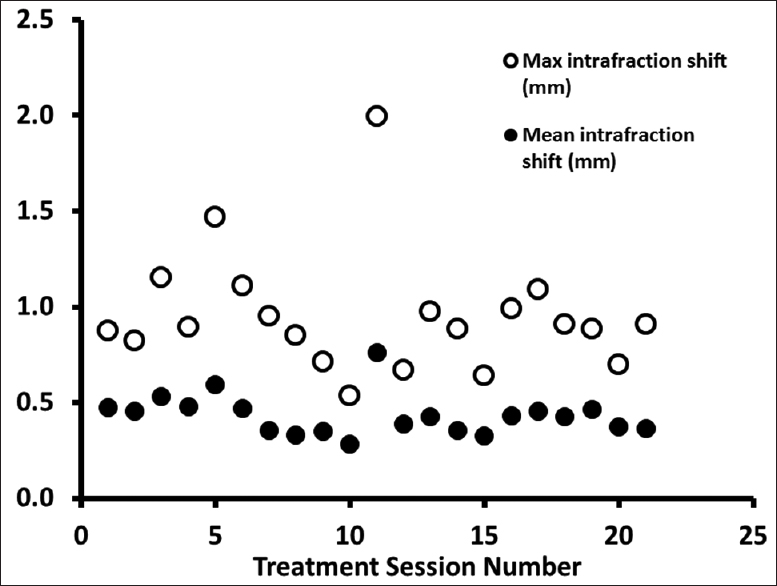
Following initial alignment, repeat X-rays were performed during the treatment delivery to verify that the patient position was maintained. The mean intrafraction shift was 0.43 mm (0.28–0.76 mm), and the maximum intrafraction shift was 0.95 mm (0.53–1.99 mm) [
Outcomes
With a median follow-up of 32 months, the response to the frameless IGRS treatment was based on the BNI score determined at follow-up appointments with the neurosurgeon and/or radiation oncologist. Good-to-excellent results were defined as a BNI score of I–III. Of the 20 patients who underwent frameless IGRS evaluated at their last follow-up, 1 (5%) patient had a post SRS BNI score of V due to failure of the frameless IGRS procedure. Eleven (55%) patients had a BNI of III as they continued to consume their TN medications following frameless SRS. One patient had occasional pain without needing medication and, therefore, was a BNI II. Seven (35%) patients scored a BNI of I as they had attained complete improvement of their symptoms and had discontinued all medications for TN. Thus, 19 (95%) of the 20 patients had a BNI of III or less at last follow-up.
The qualitative change in BNI score was marked in 7 (35%) patients, moderate in 7 (35%), and some/none/worse in 6 (30%) patients. A univariate analysis was performed to determine if any clinical factors were associated with outcomes from SRS [
Complications
No complications were reported by the TN patients. Physical examination performed by the neurosurgeon following the frameless IGRS procedure detected no new sensation or motor abnormalities.
Salvage treatments
Six patients required a salvage procedure after the frameless IGRS. Two underwent an MVD after the frameless IGRS. The first patient underwent the MVD 1 month after the frameless IGRS and attained complete pain relief 7 months after the MVD. Nine months later, he experienced a recurrence of his facial pain requiring medications. He continued to consume medications at the last follow-up 12 months later. Of note, this patient had undergone a rhizotomy prior to the frameless IGRS. Another patient underwent an MVD 13 months after the frameless IGRS and had complete pain relief 1 month later. One patient underwent a second frameless IGRS 5 months after the first one, receiving 85 Gy at each procedure. He experienced complete pain relief 2 months later. Another patient had a rhizotomy 10 months after the initial frameless IGRS and continued to require antiepileptic medications at last follow-up 22 months later. One patient underwent a rhizotomy 39 months after the initial frameless IGRS. Interestingly, this patient had undergone two rhizotomies and one MVD prior to the frameless IGRS. The final patient experienced increased pain following the frameless IGRS procedure and was treated with an increased dosage of antiepileptic medications.
The two additional patients who underwent a procedure for TN prior to the frameless IGRS were both assigned a BNI of III at the last follow-up indicating that they continued to consume TN medications.
DISCUSSION
Since the advent of SRS techniques in the treatment of TN, the most common method utilized has been the gamma knife procedure.[
The majority of LINAC SRS studies for TN have used a frame-based system with either a Novalis, Varian, or Primus device,[
Frameless radiosurgery has recently gained in popularity because it is a true noninvasive procedure without the need for anesthetics and sterile processing.[
Frameless IGRS has rarely been used to treat TN.[
The findings of the present study concur with those of Chen et al. using frameless IGRS to treat patients with TN.[
CONCLUSION
The present study describes our initial experience with treatment of TN using a frameless IGRS procedure in a multidisciplinary setting. Our data suggest that it is an effective and noninvasive method of treating TN, especially in older individuals who are refractory to medical management. The slice thickness of 0.6 mm provided increased resolution at the target compared to previous studies in the literature. This small thickness coupled with the mapping of the patient's movement during treatment allowed us to have a greater confidence to perform frameless IGRS. Further investigation is warranted with a larger sample size to confirm the efficacy of frameless IGRS for TN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge Norton Healthcare for their continued support.
References
1. Bale RJ, Laimer I, Martin A, Schlager A, Mayr C, Rieger M. Frameless stereotactic cannulation of the foramen ovale for ablative treatment of trigeminal neuralgia. Neurosurgery. 2006. 59: ONS394-ONS401
2. Bergenheim AT, Asplund P, Linderoth B. Percutaneous retrogasserian balloon compression for trigeminal neuralgia: Review of critical technical details and outcomes. World Neurosurg. 2013. 79: 359-68
3. Bozkurt M, Al-Beyati ES, Ozdemir M, Kahilogullari G, Elhan AH, Savas A. Management of bilateral trigeminal neuralgia with trigeminal radiofrequency rhizotomy: A treatment strategy for the life-long disease. Acta Neurochir. 2012. 154: 785-91
4. Brisman R. Gamma knife radiosurgery for primary management for trigeminal neuralgia. J Neurosurg. 2000. 93: 159-61
5. Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP. An analysis of the accuracy of the CyberKnife: A robotic frameless stereotactic radiosurgical system. Neurosurgery. 2003. 52: 140-6
6. Chen HI, Lee JY. The measurement of pain in patients with trigeminal neuralgia. Clin Neurosurg. 2010. 57: 129-33
7. Chen JC, Greathouse HE, Girvigian MR, Miller MJ, Liu A, Rahimian J. Prognostic factors for radiosurgery treatment of trigeminal neuralgia. Neurosurgery. 2008. 62: A53-A60
8. Chen JC, Rahimian J, Rahimian R, Arellano A, Miller MJ, Girvigian MR. Frameless image-guided radiosurgery for initial treatment of typical trigeminal neuralgia. World Neurosurg. 2010. 74: 538-43
9. Dos Santos MA, Perez de Salcedo JB, Gutierrez Diaz JA, Nagore G, Calvo FA, Samblas J. Outcome for patients with essential trigeminal neuralgia treated with linear accelerator stereotactic radiosurgery. Stereotact Funct Neurosurg. 2011. 89: 220-5
10. Dvorak T, Finn A, Price LL, Mignano JE, Fitzek MM, Wu JK. Retreatment of trigeminal neuralgia with Gamma Knife radiosurgery: Is there an appropriate cumulative dose? Clinical article. J Neurosurg. 2009. 111: 359-64
11. Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: Definition and classification. Neurosurg Focus. 2005. 18: E3-
12. Frighetto L, De Salles AA, Smith ZA, Goss B, Selch M, Solberg T. Noninvasive linear accelerator radiosurgery as the primary treatment for trigeminal neuralgia. Neurology. 2004. 62: 660-2
13. Goss BW, Frighetto L, DeSalles AA, Smith Z, Solberg T, Selch M. Linear accelerator radiosurgery using 90 gray for essential trigeminal neuralgia: Results and dose volume histogram analysis. Neurosurgery. 2003. 53: 823-8
14. Guo WY, Chu WC, Wu MC, Chung WY, Gwan WP, Lee YL. An evaluation of the accuracy of magnetic-resonance-guided Gamma Knife surgery. Stereotact Funct Neurosurg. 1996. 66: 85-92
15. . Headache Classification Committee of the International Headache Society (IHS). The International Classification of headache Disorders, 3rd edition (beta version). Cephalalgia. 2013. 33: 629-808
16. Kondziolka D, Lunsford LD, Flickinger JC, Young RF, Vermeulen S, Duma CM. Stereotactic radiosurgery for trigeminal neuralgia: A multiinstitutional study using the gamma unit. J Neurosurg. 1996. 84: 940-5
17. Kouzounias K, Schechtmann G, Lind G, Winter J, Linderoth B. Factors that influence outcome of percutaneous balloon compression in the treatment of trigeminal neuralgia. Neurosurgery. 2010. 67: 925-34
18. Kubicek GJ, Hall WA, Orner JB, Gerbi BJ, Dusenbery KE. Long-term follow-up of trigeminal neuralgia treatment using a linear accelerator. Stereotact Funct Neurosurg. 2004. 82: 244-9
19. Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand. 1971. 137: 311-4
20. Love S, Coakham HB. Trigeminal neuralgia: Pathology and pathogenesis. Brain. 2001. 124: 2347-60
21. Lucas JT, Nida AM, Isom S, Marshall K, Bourland JD, Laxton AW. Predictive nomogram for the durability of pain relief from gamma knife radiation surgery in the treatment of trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2014. 89: 120-6
22. Marshall K, Chan MD, McCoy TP, Aubuchon AC, Bourland JD, McMullen KP. Predictive variables for the successful treatment of trigeminal neuralgia with gamma knife radiosurgery. Neurosurgery. 2012. 70: 566-72
23. Pollock BE, Schoeberl KA. Prospective comparison of posterior fossa exploration and stereotactic radiosurgery dorsal root entry zone target as primary surgery for patients with idiopathic trigeminal neuralgia. Neurosurgery. 2010. 67: 633-8
24. Pusztaszeri M, Villemure JG, Regli L, Do HP, Pica A. Radiosurgery for trigeminal neuralgia using a linear accelerator with BrainLab system: Report on initial experience in Lausanne, Switzerland. Swiss Med Wkly. 2007. 137: 682-6
25. Reddy VK, Parker SL, Patrawala SA, Lockney DT, Su PF, Mericle RA. Microvascular decompression for classic trigeminal neuralgia: Determination of minimum clinically important difference in pain improvement for patient reported outcomes. Neurosurgery. 2013. 72: 749-54
26. Richards GM, Bradley KA, Tome WA, Bentzen SM, Resnick DK, Mehta MP. Linear accelerator radiosurgery for trigeminal neuralgia. Neurosurgery. 2005. 57: 1193-200
27. Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: The initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000. 47: 1013-9
28. Sandel T, Eide PK. Long-term results of microvascular decompression for trigeminal neuralgia and hemifacial spasms according to preoperative symptomatology. Acta Neurochir. 2013. 155: 1681-92
29. Smith ZA, De Salles AA, Frighetto L, Goss B, Lee SP, Selch M. Dedicated linear accelerator radiosurgery for the treatment of trigeminal neuralgia. J Neurosurg. 2003. 99: 511-6
30. Smith ZA, Gorgulho AA, Bezrukiy N, McArthur D, Agazaryan N, Selch MT. Dedicated linear accelerator radiosurgery for trigeminal neuralgia: A single-center experience in 179 patients with varied dose prescriptions and treatment plans. Int J Radiat Oncol Biol Phys. 2011. 81: 225-31
31. Stomal-Slowinska M, Slowinski J, Lee TK, Uitti RJ, Deen HG, Reimer R. Correlation of clinical findings and results of percutaneous balloon compression for patients with trigeminal neuralgia. Clin Neurol Neurosurg. 2011. 113: 14-21
32. Varela-Lema L, Lopez-Garcia M, Maceira-Rozas M, Munoz-Garzon V. Linear accelerator stereotactic radiosurgery for trigeminal neuralgia. Pain Physician. 2015. 18: 15-27
33. Verbakel WF, Lagerwaard FJ, Verduin AJ, Heukelom S, Slotman BJ, Cuijpers JP. The accuracy of frameless stereotactic intracranial radiosurgery. Radiother Oncol. 2010. 97: 390-4
34. Wurm RE, Erbel S, Schwenkert I, Gum F, Agaoglu D, Schild R. Novalis frameless image-guided noninvasive radiosurgery: Initial experience. Neurosurgery. 2008. 62: A11-A17
35. Zahra H, Teh BS, Paulino AC, Yoshor D, Trask T, Baskin D. Stereotactic radiosurgery for trigeminal neuralgia utilizing the BrainLAB Novalis system. Technol Cancer Res Treat. 2009. 8: 407-12
36. Zhang H, Lei D, You C, Mao BY, Wu B, Fang Y. The long-term outcome predictors of pure microvascular decompression for primary trigeminal neuralgia. World Neurosurg. 2013. 79: 756-62