- Department of Neurosurgery, Royal Victoria Infirmary, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, United Kingdom,
- Department of General and Special Surgery, Faculty of Medicine, The Hashemite University, Zarqa, Jordan, London, United Kingdom.
- Wessex Spinal Unit, University Hospital Southampton NHS Foundation Trust, Southampton, London, United Kingdom.
- Department of Neurosurgery, St George’s University Hospitals NHS Foundation, London, United Kingdom.
Correspondence Address:
Alaa Al-Mousa
Department of Neurosurgery, St George’s University Hospitals NHS Foundation, London, United Kingdom.
DOI:10.25259/SNI_151_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: B. Ashan P. Jayasekera1, Alaa Al-Mousa2, Anan Shtaya3, Erlick Pereira4. Freehand external ventricular drain insertion – is there a learning curve?. 26-Apr-2021;12:193
How to cite this URL: B. Ashan P. Jayasekera1, Alaa Al-Mousa2, Anan Shtaya3, Erlick Pereira4. Freehand external ventricular drain insertion – is there a learning curve?. 26-Apr-2021;12:193. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10750
Abstract
Background: Accuracy of freehand insertion of external ventricular drains (EVDs) is influenced by many factors including etiology and presence of midline shift. We sought to assess if junior neurosurgical trainees’ performance in accurately inserting EVDs improves with experience, using a radiological grading system.
Methods: EVD insertion procedures from the first 3 years of training were identified from the operative logbooks of three trainees. Postoperative CT head scans were graded for accuracy of placement and intraventricular catheter length.
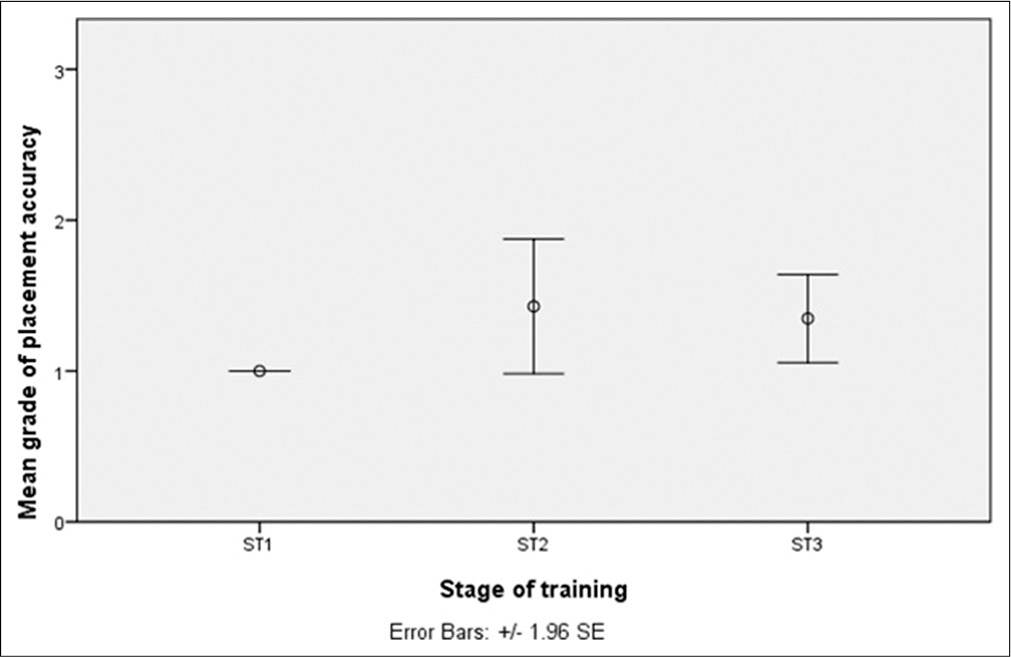
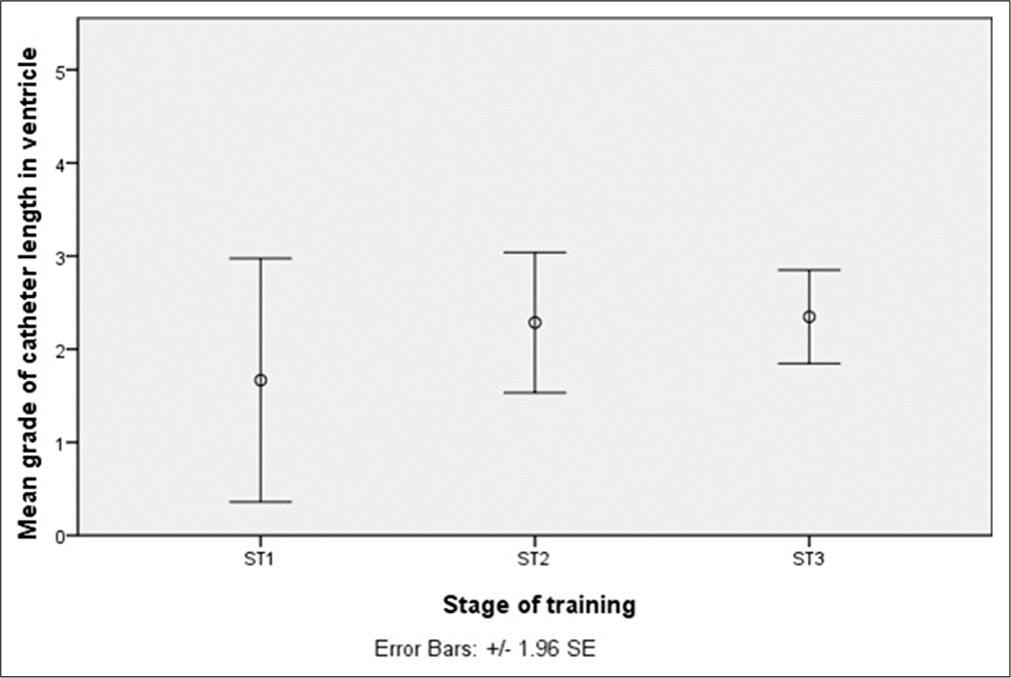
Results: 40 frontal EVDs performed primarily by the trainees were identified, after 34 assists, revision surgeries, parietal, or occipital insertions were excluded from the study. The mean number (±1 SD) of procedures was 7.7 ± 4.5 at ST3, 4.7 ± 2.5 at ST2, and 1 ± 1 at ST1. About 80% of EVDs were optimally inserted. There was no statistically significant difference in placement accuracy between the three training grades (P = 0.669), nor any difference in intraventricular catheter length (P = 0.697). There were no statistically significant differences between surgeons’ accuracy at each grade.
Conclusion: We report good accuracy of EVDs tip position inserted by junior neurosurgery trainees. Trainees perform more procedures independently as they progress in their career. Further studies including senior years of training performance, other procedure factors and outcome should be considered.
Keywords: Accuracy, Education, External ventricular drain, Learning curve, Training, Ventriculostomy
INTRODUCTION
External ventricular drain (EVD) insertion is a common neurosurgical procedure, often performed by junior neurosurgical trainees. EVDs measure intracranial pressure, divert cerebrospinal fluid in management of hydrocephalus and raised intracranial pressure, and allow for intrathecal administration of pharmacologic agents. Freehand insertion of EVDs using anatomical landmarks is considered the primary method for placement, although alternative techniques have shown improved accuracy in positioning.[
Kakarla et al. examined postoperative computed tomographic (CT) head scans, in 346 patients who underwent freehand bedside ventriculostomy, using a radiological grading system to assess for accuracy of placement.[
Simulated ventriculostomy procedures to circumvent the issues of patient safety, limited trainee working hours, and theater time have been developed.[
To assess whether there is a procedural learning curve in EVD placement, we sought to examine whether accuracy of placement of external ventricular catheters improves with experience in junior neurosurgical trainees from specialty training years one (ST1) to three (ST3), using a radiological grading system.
MATERIALS AND METHODS
Patient details for all EVDs inserted during the first 3 years (ST1-ST3) of each neurosurgical trainee’s training were identified from the operative logbooks of three neurosurgical trainees. As the three trainees were at different grades, operations spanned 5 years from August 2006 to July 2011 inclusive. Cases where the surgeon was assisting or observing only and revision procedures were excluded from the analysis, as were occipital and parietal EVDs. Only first surgery frontal EVDs primarily performed by the trainees were included in the study. Details regarding etiology and presentation were collected from case notes.
Preoperative and postoperative CT head scans were obtained from the hospital Centricity Picture Archiving and Communications (PACS) and Clear Canvas Workstation systems. The most recent CT head scan before the date of EVD insertion was used as the preoperative scan for analysis. The first scan following the procedure was used as the postoperative scan for analysis. Hospital operating theater logbooks were checked to ensure that the EVD had not been revised in the interim.
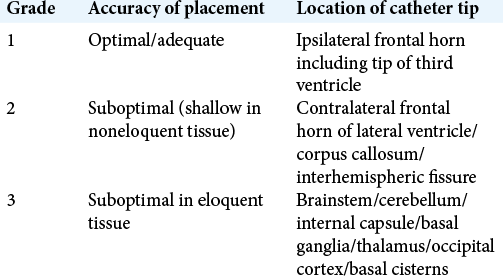
Accuracy of ventricular catheter placement was assessed using the grading system described and validated by Kakarla et al. [
Table 1:
Radiological grading system for accuracy of placement, after Kakarla et al.[
Table 2:
Radiological grading system for intraventricular catheter length, after Wan et al.[
We compared accuracy and intra-ventricular length across the ST1, ST2, and ST3 grades by analysis of variance with PASW statistics 18.0.
RESULTS
40 frontal EVDs were performed primarily by the three trainees during the first 3 years of their training. 74 procedures were identified in total, but assistance, revision surgery, parietal, and occipital insertions were excluded from the study (n = 34). No procedures utilized image guidance or framed stereotaxy as all were performed freehand. The mean number (±1 SD) of procedures was 7.7± 4.5 at ST3, 4.7 ± 2.5 at ST2, and 1 ± 1 at ST1. The total number of procedures at each grade was 23 at ST3, 14 at ST2, and 3 at ST1. Etiology included SAH (n = 22), posterior fossa hemorrhage/infarct (n = 5), supratentorial intracerebral hemorrhage (n = 4), posterior fossa tumor/surgery (n = 5), intra-ventricular tumor (n = 2), meningitis (n = 1), and trauma (n = 1).
About 80% of EVDs were optimally inserted. 32 catheters were Grade 1 in placement, two Grade 2 and six Grade 3 [
The mean (±1 SD) length of catheter within the ventricle was 23 mm ± 9.5. There was no significant difference in intra-ventricular catheter length between the three training grades [P = 0.697,
DISCUSSION
The freehand insertion of an EVD is often the remit of junior neurosurgical trainees. Indeed, in the authors’ experience, alongside burr-hole drainage of chronic subdural hematomas, it is often one of the first neurosurgical operations performed by a trainee. Despite being a well-established treatment for hydrocephalus, published studies evaluating the success of placement by freehand insertion vary greatly in their estimates of surgical accuracy. For instance, Toma et al. reported accurate placement of EVD’s in only 73 out of 183 procedures (39.9%).[
Recently, three-dimensional volumetric reconstruction of CT scans has also been used with mapping of virtual EVD trajectories to suggest that trajectories perpendicular to the skull or using the surface landmark of contralateral medial canthus are superior to ipsilateral medial canthus for determining the optimal freehand approach for cannulating the ventricles in a small study of ten patients, although a learning curve was not able to be assessed.[
Here, we utilized the previously described grading systems of Kakarla et al.[
With the learning of any new procedure, performance is anticipated to improve with experience. Plotting such improvements with experience produces a so-called “learning curve.” In the context of surgery, performance can be measured through quantitative measures of surgical processes such as time taken to perform a procedure, extent of resection, or blood loss, or clinical outcomes such as length of stay, morbidity, and mortality.[
CONCLUSION
We report good accuracy of EVDs inserted by junior neurosurgery trainees in the early years of their training in a busy neurosurgery unit. Trainees perform more procedures independently as they progress in their career. Although there was no significant improvement in placement accuracy and intra-ventricular length of EVDs with experience, the number of EVDs inserted in the 1st year of training was small to perform meaningful statistical analysis. To attain a certificate of completion of training within the United Kingdom for neurosurgery, minimum quotas of index cases at varying supervision levels have been suggested. The majority are cranial micro neurosurgical operations such as clipping a cerebral aneurysm which clearly exhibits a learning curve.[
British neurosurgical trainees’ complete workplace-based assessments (WBAs) both in the domains of procedure-based assessments and direct observations of procedural skill (DOPS) within their intercollegiate surgical curriculum program. These WBAs remain of questionable validity a decade since their introduction and objective quantitative measures of procedural success and error detection may prove more valid.[
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge sharing of data from surgical logbooks of Mr Martin Gillies and Mr Tim Lawrence.
References
1. Alaraj A, Lemole MG, Finkle JH, Yudkowsky R, Wallace A, Luciano C. Virtual reality training in neurosurgery: Review of current status and future applications. Surg Neurol Int. 2011. 2: 52
2. Banerjee PP, Luciano CJ, Lemole GM, Charbel FT, Oh MY. Accuracy of ventriculostomy catheter placement using a head and hand-tracked high-resolution virtual reality simulator with haptic feedback. J Neurosurg. 2007. 107: 515-21
3. Bann S, Khan M, Datta V, Darzi A. Surgical skill is predicted by the ability to detect errors. Am J Surg. 2005. 189: 412-5
4. Cenydd LA, John NW, Phillips NI, Gray WP. VCath: A tablet-based neurosurgery training tool. Stud Health Technol Inform. 2013. 184: 20-3
5. Champion HR, Meglan DA, Shair EK. Minimizing surgical error by incorporating objective assessment into surgical education. J Am Coll Surg. 2008. 207: 284-91
6. Fried MP, Satava R, Weghorst S, Gallagher AG, Sasaki C, Ross D. Identifying and reducing errors with surgical simulation. Qual Saf Health Care. 2004. 13: i19-26
7. Hopper AN, Jamison MH, Lewis WG. Learning curves in surgical practice. Postgr Med J. 2007. 83: 777-9
8. Hsieh CT, Chen GJ, Ma HI, Chang CF, Cheng CM, Su YH. The misplacement of external ventricular drain by freehand method in emergent neurosurgery. Acta Neurol Belg. 2011. 111: 22-8
9. Kakarla UK, Kim LJ, Chang SW, Theodore N, Spetzler RF. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery. 2008. 63: ONS162-166
10. Krombach G, Ganser A, Fricke C, Rohde V, Reinges M, Gilsbach J. Virtual placement of frontal ventricular catheters using frameless neuronavigation: An “unbloody training” for young neurosurgeons. Minim Invasive Neurosurg. 2000. 43: 171-5
11. Lemole GM, Banerjee PP, Luciano C, Neckrysh S, Charbel FT. Virtual reality in neurosurgical education: Part-task ventriculostomy simulation with dynamic visual and haptic feedback. Neurosurgery. 2007. 61: 142-8
12. Lind CR, Tsai AM, Lind CJ, Tsai AM, Lind CJ, Law AJ. Ventricular catheter placement accuracy in non-stereotactic shunt surgery for hydrocephalus. J Clin Neurosci. 2009. 16: 918-20
13. Maurice-Williams RS, Kitchen ND. Ruptured intracranial aneurysms--learning from experience. Br J Neurosurg. 1994. 8: 519-27
14. Muirhead WR, Basu S. Trajectories for frontal external ventricular drain placement: Virtual cannulation of adults with acute hydrocephalus. Br J Neurosurg. 2012. 26: 710-6
15. Nowacki A, Wagner F, Soll N, Hakim A, Beck J, Raabe A. Preliminary results of emergency computed tomography-guided ventricular drain placement-precision for the most difficult cases. World Neurosurg. 2018. 114: e1290-6
16. Pereira EA, Dean BJ. British surgeons’ experiences of mandatory online workplace-based assessment. J R Soc Med. 2009. 102: 287-93
17. Pereira EA, Dean BJ. British surgeons’ experiences of a mandatory online workplace based assessment portfolio resurveyed three years on. J Surg Educ. 2013. 70: 59-67
18. Shtaya A, Roach J, Sadek AR, Gaastra B, Hempenstall J, Bulters D. Image guidance and improved accuracy of external ventricular drain tip position particularly in patients with small ventricles. J Neurosurg. 2018. 2018: 1-8
19. Toma AK, Camp S, Watkins LD, Grieve J, Kitchen ND. External ventricular drain insertion accuracy: Is there a need for change in practice?. Neurosurgery. 2009. 65: 1197-1200
20. Wan KR, Toy JA, Wolfe R, Danks A. Factors affecting the accuracy of ventricular catheter placement. J Clin Neurosci. 2011. 18: 485-8
21. Yudkowsky R, Luciano C, Banerjee P, Schwartz A, Alaraj A, Lemole Gm. Practice on an augmented reality/haptic simulator and library of virtual brains improves residents’ ability to perform a ventriculostomy. Simul Healthc. 2013. 8: 25-31