- Departments of Neurosurgery, Hospital Angeles Pedregal, Mexico City, Mexico.
- Departments of Neurology, Hospital Angeles Pedregal, Mexico City, Mexico.
- Departments of Diagnostic and Therapeutic Radiology Hospital Angeles Pedregal, Mexico City, Mexico.
- Departments of Neuroanesthesiology, Hospital Angeles Pedregal, Mexico City, Mexico.
Correspondence Address:
Luis Alberto Ortega-Porcayo
Departments of Neuroanesthesiology, Hospital Angeles Pedregal, Mexico City, Mexico.
DOI:10.25259/SNI_401_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luis Alberto Ortega-Porcayo1, Eduardo Perusquia Ortega2, Oscar Quiroz-Castro3, Roger Antonio Carrillo-Meza3, Juan Antonio Ponce-Gomez1, Samuel Romano-Feinholz1, Victor Alcocer-Barradas1, Alfredo Ramirez-Gutierrez de Velasco3, Marcela Osuna Zazueta4. Frontotemporal brain sagging syndrome: Craniospinal hypovolemia secondary to a T6-T7 cerebrospinal fluid-venous fistula. 15-Aug-2020;11:250
How to cite this URL: Luis Alberto Ortega-Porcayo1, Eduardo Perusquia Ortega2, Oscar Quiroz-Castro3, Roger Antonio Carrillo-Meza3, Juan Antonio Ponce-Gomez1, Samuel Romano-Feinholz1, Victor Alcocer-Barradas1, Alfredo Ramirez-Gutierrez de Velasco3, Marcela Osuna Zazueta4. Frontotemporal brain sagging syndrome: Craniospinal hypovolemia secondary to a T6-T7 cerebrospinal fluid-venous fistula. 15-Aug-2020;11:250. Available from: https://surgicalneurologyint.com/surgicalint-articles/10207/
Abstract
Background: The frontotemporal brain sagging syndrome (FTBSS) is defined as an insidious/progressive decline in behavior and executive functions, hypersomnolence, and orthostatic headaches attributed to cerebrospinal fluid (CSF) hypovolemia. Here, a T6 CSF-venous fistula (e.g., between the subarachnoid CSF and a paraspinal vein) resulted in a CSF leak responsible for craniospinal hypovolemia.
Case Description: A 56-year-old male started with orthostatic headaches and fatigue after scuba diving. His symptoms included progressive, vertigo, tinnitus, nausea, lack of judgment, inappropriate behavior, memory dysfunction, apathy, tremor, orofacial dyskinesia, dysarthria, dysphagia, and hypersomnolence. The lumbar puncture revealed an opening pressure of 0 cm H2O. Magnetic resonance imaging (MRI) findings included brain sagging, bilateral temporal lobe herniation, and pachymeningeal enhancement. The computed tomography (CT) myelogram showed a thoracic diverticulum and a CSF-venous leak at the T6-T7 level. Surgery, which comprised a T6-T7 laminotomy, allowed for dissecting, clipping, and ligating the diverticulum/fistula. The patient improved postoperatively (e.g., cognitive, behavioral, and brainstem symptoms). The follow-up MRI’s showed the reversion of the sagging index/uncal herniation.
Conclusion: The FTBSS should be considered in the differential diagnosis of an early onset frontotemporal dementia. Establishing the diagnosis and localizing the site of a spinal CSF/venous leak warrant both MRI and myelogram CT studies, to pinpoint the CSF leak site for proper surgical clipping/ligation of these thoracic diverticulum/CSF-venous leaks.
Keywords: Cerebrospinal fluid-venous fistula, Craniospinal hypovolemia, Frontotemporal brain sagging syndrome, Intracranial hypotension
INTRODUCTION
Spontaneous intracranial hypotension (SIH) is characterized by orthostatic headaches that result from low cerebrospinal fluid (CSF) pressure, accompanied by neck stiffness and hearing deficits.[
CASE REPORT
A 56-year-old male presented in 2002 with severe orthostatic bifrontal headaches and fatigue after performing scuba diving. From 2002 to 2005, his behavior was inappropriate and he was diagnosed with major depression. For and by 2009, he continued with orthostatic headaches plus lumbar pain, vertigo, tinnitus, nausea, and once vomiting. By 2015, he complained of memory dysfunction, apathy, neck pain, severe fatigue, and orthostatic headaches; he was admitted to the hospital with a suspected viral meningoencephalitis and was treated with antivirals, antibiotics, and levetiracetam with minimal improvement. Later in that year, he was given a short cycle of prednisolone and showed partial improvement. From 2016 to 2018, his symptoms exacerbated, and he developed hypersomnolence, sleep apnea, memory impairment, inappropriate behavior, bradypsychia, bradylalia, hand and oral automatisms, urinary urgency, neck pain, ataxia, tremor, dysarthric speech, and dysphagia. We admitted him in 2018 after worsening of the state of consciousness, memory impairment, and inappropriate behavior.
His physical examination was normal, but the neurological examination revealed fluctuating consciousness (normal to clouded), inattention, disorientation, disinhibited behavior, impaired judgment, bradylalia, and memory impairment. On fundoscopy, there was blurring of the medial optic margins, elevation of the optic disc, and loss of venous pulsations (II). Bilateral absent gag reflex, flattening of the palatal arch (IX, X), dysphonia (X), hypotrophy, and fasciculations of the tongue (XII). Motor strength was 4/5 bilaterally with generalized hyperreflexia and both rest and intention tremor.
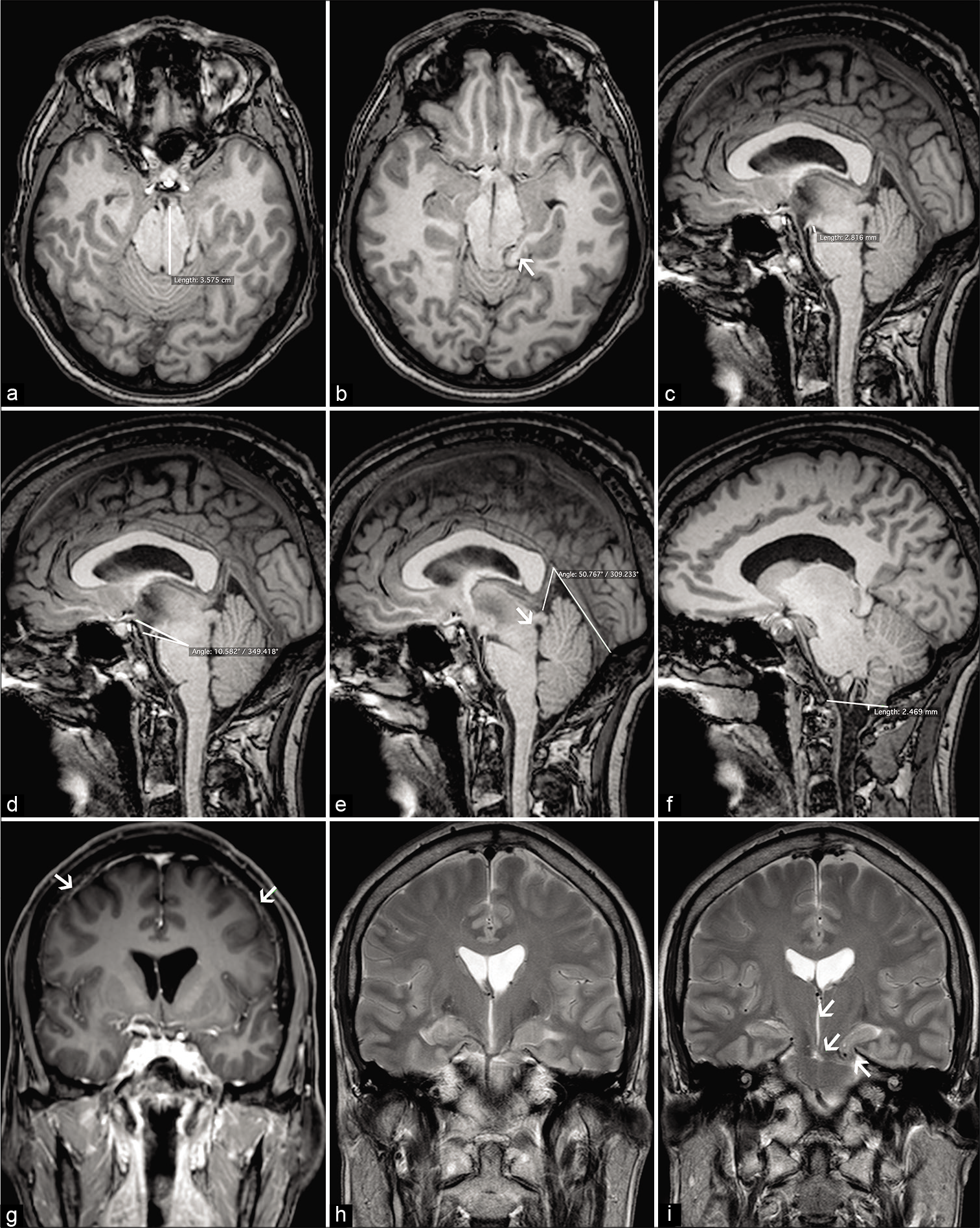
Neuropsychological test battery (e.g., MoCA, WAIS, CPT, Tower of London test, TAVEC, WCST, and Rey complex figure test) was all abnormal, which showed mild deficits in attention and planning, plus severe deficits in working, explicit, and episodic memory. A lumbar puncture revealed an opening pressure of 0 cm H2O, but normal CSF laboratory findings. The electroencephalography showed dominant background alpha rhythm, left occipital amplitude asymmetry without epileptic activity. The brain MRI showed severe brain sagging, minimal cerebellar tonsillar descent, bilateral temporal lobe herniation, and pachymeningeal enhancement [
Figure 1:
Preoperative brain magnetic resonance imaging showed severe brain sagging signs, in the axial plane (a) a distorted midbrain anatomy was observed at the level of the tentorial incisura in which an anteroposterior midbrain elongation (35 mm) and temporal lobe herniation were evident. Notice the anterior and posterior parahippocampal herniation (b). In the sagittal plane, there was sagging of the brainstem, cerebellar tonsillar descent, shortened pontomammillary distance (2.8 mm/abnormal <5.5 mm) (c), flattening of the pontomesencephalic angle (10.5°/abnormal <50°) (d), narrow vein of Galen/straight sinus angle (50.7°), aqueduct displacement (e), and cerebellar tonsillar descent using the McRae line (2.4 mm/abnormal >5 mm) (f). Notice the generalized pachymeningeal enhancement (g). In the coronal plane, the third ventricle was thin and elongated, the mammillothalamic tract was displaced downwardly, and bilateral uncal herniation was observed (h and i).[2]
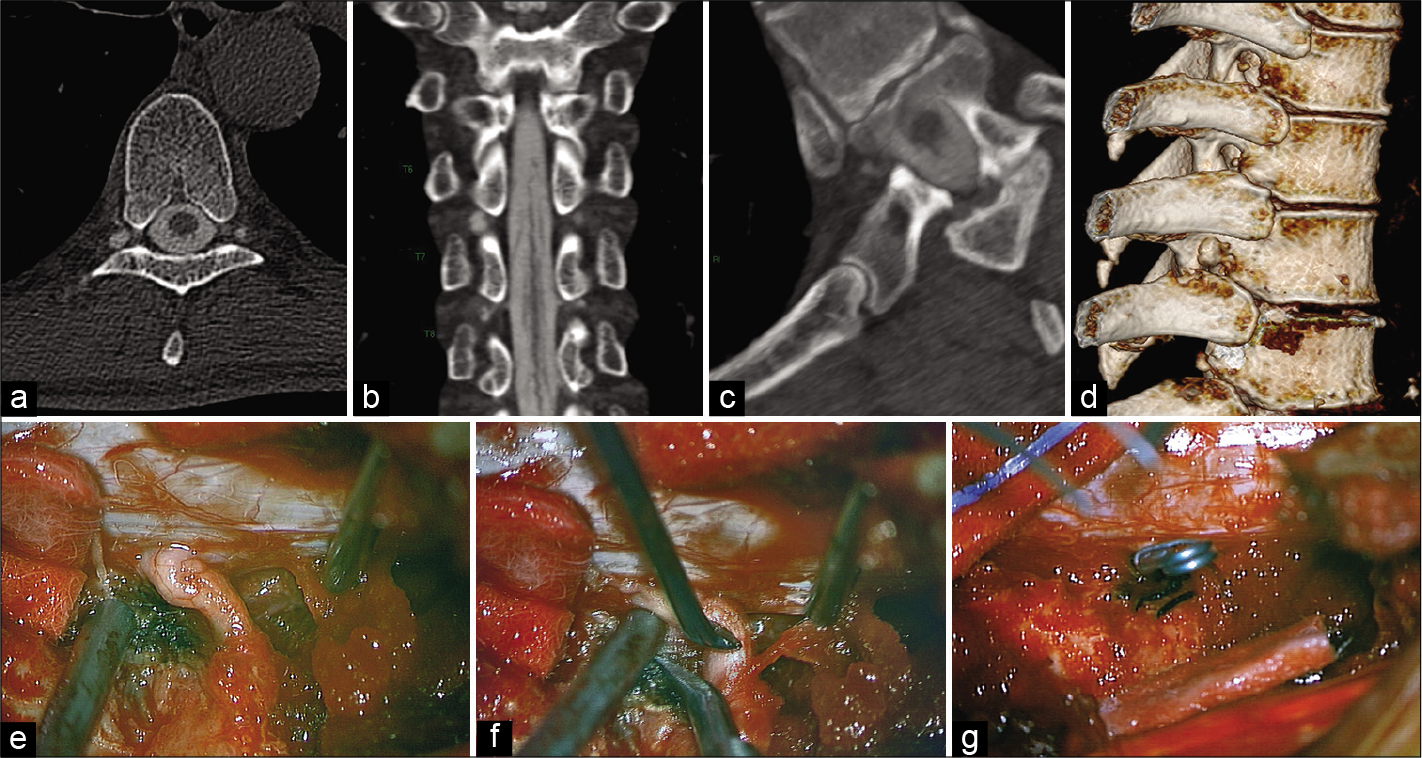
The thoracic MRI showed multiple diverticula. A computed tomography (CT) myelogram performed in prone, supine, and lateral positions revealed a right T6 thoracic meningeal diverticulum associated to a CSF-venous leak and a T7 small meningeal diverticulum [
Figure 2:
Hyperdense paraspinal vein sign[6,18] was observed on computed tomography (CT) myelogram. A curvilinear high attenuation vascular structure was observed in connection with a T7 nerve root diverticulum (a and b). The contrast filling paraspinal vein and CSF leak was better observed on CT myelography in the prone and right lateral decubitus position. Notice the paraspinal vein (c) and the 3D reconstruction of the thoracic diverticula (d). CSF-venous leak was localized, and the thoracic roots were exposed (e). Multiples veins were coagulated (f) during the root dissection; vascular Sugita straight clips were placed at the origin of the root sleeves (g).
Surgical technique
A two-level T6-T7 laminotomy was performed to address the CSF-venous leak (e.g., using fluoroscopic and neuronavigation). The T6 and T7 nerve roots were exposed, the diverticulum was isolated/dissected and ligated from the surrounding veins (e.g., ligated with vascular Sugita straight clips placed at the origin of the root sleeves) [
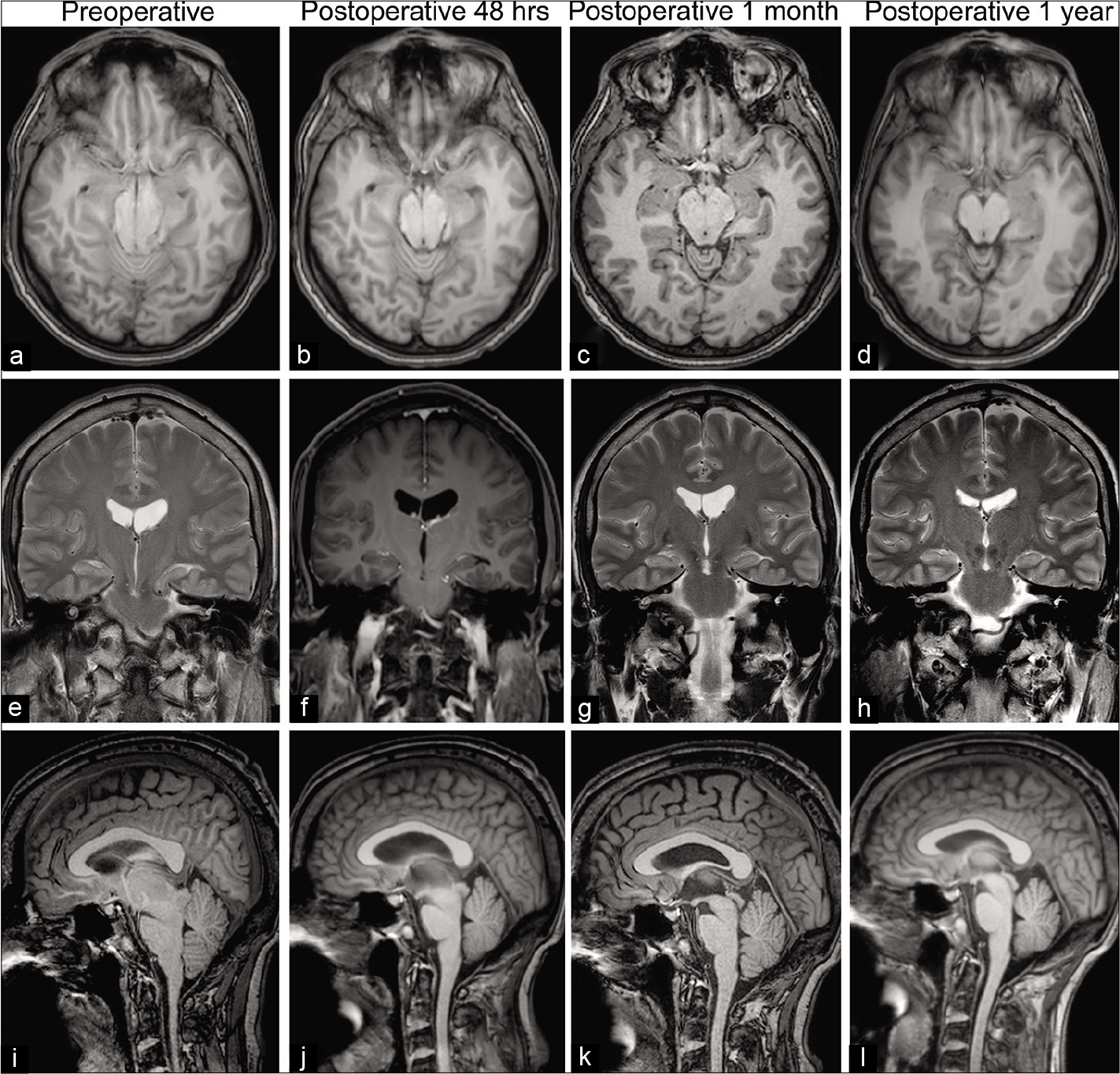
One year later, cognitive, behavioral, and cranial nerve symptoms progressively improved; now 2 years postoperatively, he remains asymptomatic. Neuropsychological tests, 6 months and 1 year after surgery, revealed significant improvement. Sleep apnea improved, but he still requires continuous positive airway pressure. The postoperative MRI’s (48 h, 1 month, and 12 months after surgery) showed progressive improvement of the sagging index/uncal herniation [
Figure 3:
MRI showed axial, coronal, and sagittal planes (a-l). Notice the progressive hypotension resolution over the time. Anterior-posterior midbrain diameter was 35 mm preoperative versus 25 mm postoperative (1 year). Pontomammillary distance was 2.8 mm preoperative versus 8.1 mm postoperative (1 year). Pontomesencephalic angle was 10.5° preoperative versus 51° postoperative (1 year). Vein of Galen/straight sinus angle was 50.7° preoperative versus 68.1° postoperative. Sagging index (anterior-posterior midbrain diameter/pontomammillary distance) improved significantly 1 year after surgery (12.5 vs. 3.08). A sagging index higher than 10 suggests an atypical clinical presentation for craniospinal hypovolemia.[1]
DISCUSSION
In 2002, Hong et al.[
Although SIH, also called craniospinal hypovolemia, is caused by leakage of CSF volume from the spine, CSF pressure is normal in the majority of patients and it only decreases when the rate of CSF volume loss cannot be furthered compensated.[
Etiology of FTBSS
The etiology of FTBSS is usually attributed to a spontaneous spinal CSF leak, however in the largest clinical series, 12/29 (41%) cases had normal spine imaging.[
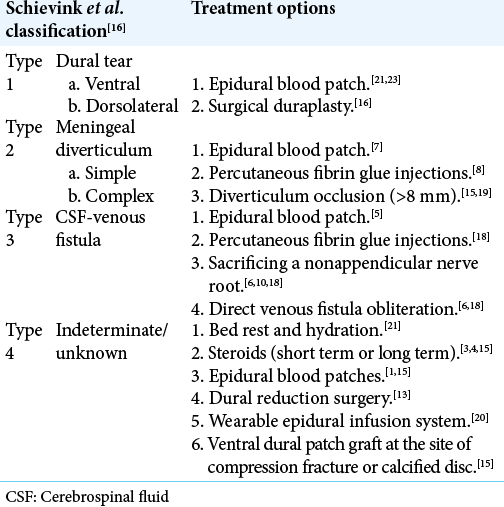
Treatment options
Treatment options for FTBSS depend on the CSF leak type [
CONCLUSION
We should consider FTBSS in the differential diagnosis of an early onset FTD. Unfortunately, the CSF leak is rarely detected early in the clinical course. In this case, MR/myelo- CT was essential to identify the T6-T7 CSF-venous fistula that was appropriately surgically dissected/ligated and should be similarly used in other comparable cases.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Capizzano AA, Lai L, Kim J, Rizzo M, Gray L, Smoot MK. Atypical presentations of intracranial hypotension: Comparison with classic spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2016. 37: 1256-61
2. Choi H, Lee MJ, Choi HA, Cha J, Chung CS. Intracranial structural alteration predicts treatment outcome in patients with spontaneous intracranial hypotension. Cephalalgia. 2018. 38: 323-31
3. Goto S, Ohshima T, Yamamoto T, Shimato S, Nishizawa T, Kato K. Successful steroid treatment of coma induced by severe spontaneous intracranial hypotension. Nagoya J Med Sci. 2016. 78: 229-36
4. Hong M, Shah GV, Adams KM, Turner RS, Foster NL. Spontaneous intracranial hypotension causing reversible frontotemporal dementia. Neurology. 2002. 58: 1285-7
5. Kranz PG, Amrhein TJ, Gray L. CSF venous fistulas in spontaneous intracranial hypotension: Imaging characteristics on dynamic and CT myelography. AJR Am J Roentgenol. 2017. 209: 1360-6
6. Kranz PG, Amrhein TJ, Schievink WI, Karikari IO, Gray L. The hyperdense paraspinal vein sign: A marker of CSF-Venous fistula. AJNR Am J Neuroradiol. 2016. 37: 1379-81
7. Kranz PG, Gray L, Taylor JN. CT-guided epidural blood patching of directly observed or potential leak sites for the targeted treatment of spontaneous intracranial hypotension. AJNR Am J Neuroradiol. 2011. 32: 832-8
8. Kranz PG, Malinzak MD, Amrhein TJ, Gray L. Update on the diagnosis and treatment of spontaneous intracranial hypotension. Curr Pain Headache Rep. 2017. 21: 37
9. Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension?. Cephalalgia. 2016. 36: 1209-17
10. Kumar N, Diehn FE, Carr CM, Verdoorn JT, Garza I, Luetmer PH. Spinal CSF venous fistula: A treatable etiology for CSF leaks in craniospinal hypovolemia. Neurology. 2016. 86: 2310-2
11. Miyazawa K, Shiga Y, Hasegawa T, Endoh M, Okita N, Higano S. CSF hypovolemia vs intracranial hypotension in spontaneous intracranial hypotension syndrome. Neurology. 2003. 60: 941-7
12. Mokri B. Spontaneous cerebrospinal fluid leaks: From intracranial hypotension to cerebrospinal fluid hypovolemia-evolution of a concept. Mayo Clin Proc. 1999. 74: 1113-23
13. Mostofi E, Schievink WI, Sim VL. Dural reduction surgery: A treatment option for frontotemporal brain sagging syndrome. Can J Neurol Sci. 2016. 43: 593-5
14. Sayao AL, Heran MK, Chapman K, Redekop G, Foti D. Intracranial hypotension causing reversible frontotemporal dementia and coma. Can J Neurol Sci. 2009. 36: 252-6
15. Schievink WI, Maya MM, Barnard ZR, Moser FG, Jean-Pierre S, Waxman AD. Behavioral variant frontotemporal dementia as a serious complication of spontaneous intracranial hypotension. Oper Neurosurg (Hagerstown). 2018. 15: 505-15
16. Schievink WI, Maya MM, Jean-Pierre S, Nuno M, Prasad RS, Moser FG. A classification system of spontaneous spinal CSF leaks. Neurology. 2016. 87: 673-9
17. Schievink WI, Meyer FB, Atkinson JL, Mokri B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg. 1996. 84: 598-605
18. Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology. 2014. 83: 472-3
19. Schievink WI, Reimer R, Folger WN. Surgical treatment of spontaneous intracranial hypotension associated with a spinal arachnoid diverticulum. Case report. J Neurosurg. 1994. 80: 736-9
20. Schievink WI, Rosner HL, Louy C. A wearable epidural catheter infusion system for patients with intractable spontaneous intracranial hypotension. Reg Anesth Pain Med. 2015. 40: 49-51
21. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006. 295: 2286-96
22. Wicklund MR, Mokri B, Drubach DA, Boeve BF, Parisi JE, Josephs KA. Frontotemporal brain sagging syndrome: An SIH-like presentation mimicking FTD. Neurology. 2011. 76: 1377-82
23. Wu JW, Hseu SS, Fuh JL, Lirng JF, Wang YF, Chen WT. Factors predicting response to the first epidural blood patch in spontaneous intracranial hypotension. Brain. 2017. 140: 344-52