Intracranial aneurysms can be classified according to pathogenesis, shape, or its causes. The classification according to its form is the most used and it can be divided into saccular and nonsaccular types. Fusiform aneurysms are nonsaccular dilatations that involve the vessel wall for a variable distance and it can present different formation process.[ 18 ]
Intracranial fusiform aneurysms are rare, although the number of cases has increased in recent years, mainly in young patients.[ 4 9 11 16 23 ] They represent about 3–13% of all intracranial aneurysms and they are often presented at the vertebrobasilar system.[ 16 18 ] Fusiform aneurysms in the anterior circulation most of the times affect the middle cerebral artery (MCA), followed by the internal carotid artery (ICA) and the anterior cerebral artery (ACA).[ 16 18 ] The supraclinoid segment is the site for the majority of cases of fusiform aneurysm of the intracranial ICA.[ 16 ]
Fusiform aneurysms have different underlying pathologies, hemodynamics, anatomical distributions, natural histories, and treatments compared to the saccular variety. The two principal causes for this type of aneurysm are dissection and atherosclerosis; disorders of collagen and elastin metabolism, by infections, very rarely by neoplastic invasion of the arterial wall and also iatrogenesis are other origins for this vasculopathy.[ 18 ]
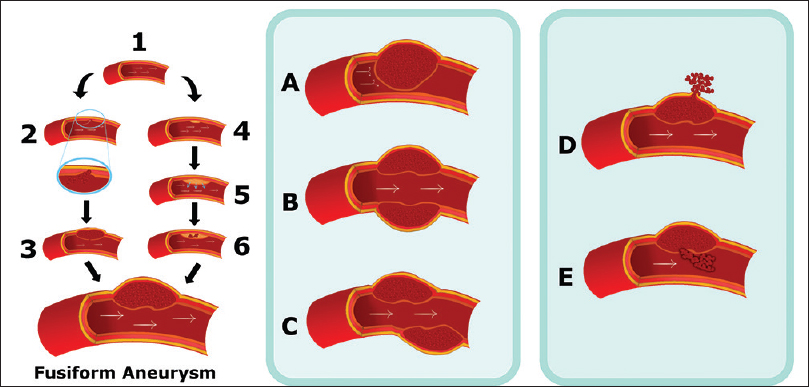
An essential feature of the intracranial fusiform aneurysms is the communication between both lumens (the true lumen and the pseudolumen) through a disrupted portion of the internal elastic lamina in most of the cases. A possibility for its evolution is the disruption advance to the adventitia, rupturing the aneurysm and causing a subarachnoid hemorrhage (SAH), or it can be contained by the media layer, which will result in ischemia or stenosis of the artery caused by the enlargement of an aneurysm toward the artery lumen.[ 17 ] A propose for atherosclerotic fusiform aneurysms pathogenesis and evolution is that the initial event in the formation of the aneurysm is a lipid deposition in and beneath the intima. This disrupts the internal elastic membrane (IEM) and infiltrates the muscular wall. Intramural hemorrhage and rupture of the atheroma lead to transmural extension of the thrombus and thicken the intima to create the fusiform shape of an aneurysm. The breach of the vasa vasorum by shear stress or by forces on the parent vessel lumen then causes intimal impairment, mainly of the IEM. This process leads to the formation of an intramural hematoma due to the bleeding into the arterial wall. If the dissection occurs between the internal elastic lamina and the media, the vessel lumen becomes narrow or occluded with an intramural hematoma and the patients present with ischemic symptoms. If dissection is located between the media and the adventitia, the rupture of an aneurysm can lead to a SAH or intracranial hemorrhage and the patient will present hemorrhage symptoms. The rupture into the vessel lumen of an intramural thrombus can cause a distal embolization. However, the further expansion of the intramural clot will lead to vessel occlusion. After occluding vessel by intramural hematoma, it can be recanalized and enlarge the dissection both laterally and longitudinally. Serpentine channel forms as disease extend longitudinally, combined with varying degrees of intraluminal thrombosis.[ 18 ] So, it's possible to classify six stages, for atherosclerotic and nonatherosclerotic fusiform aneurysms evolution, and they are arterial dissection with intramural hemorrhage between the intima and media producing a focal narrowing of vessel and rupture producing bleeding into the brain or subarachnoid space after the arterial dissection. Rupture of dissection into the arterial lumen produces a distal embolization. Further expansion of an intramural clot leads to vessel occlusion. Progress enlargement of dissection both laterally and longitudinally and serpentine channel within dissected thrombotic aneurysm,[ 18 ] all this process is exemplified in Figure 1 .
Figure 1
Model of the fusiform aneurysm pathogenesis. 1: Normal intracranial vessel. 2: Dissection in the internal elastic lamina. 3: Formation of an intramural hematoma. 4: Lipid deposition in and beneath the intima. 5: Disruption of the internal elastic membrane and infiltration to the muscular wall. 6: Intramural hemorrhage. Formation of a hematoma, leading to five main evolution patterns: A – Further expansion of the intramural hematoma. B – Progress enlargement of dissection both laterally and longitudinally. C – Serpentine channel formation. D – Rupture. E – Rupture into the arterial lumen
The radiologic features of an aneurysm can elucidate its anatomy and pathogenesis; a fusiform dolicho-vessel or a stenotic vessel secondary to intramural hemorrhage shows elements of a fusiform aneurysm and an aneurysm formed by a dissection of the vessel wall that may be increasing laterally and longitudinally assuming a “serpentine” pattern, may narrow the lumen because of the presence of a hematoma or a clot, and may rupture the wall and cause an intramural hemorrhage what can initiate distal embolic events or an aneurysm can rupture causing a SAH, for example.[ 16 ] The same processes that are responsible for the formation of an aneurysm are also responsible for an eventual rupture, despite the hemorrhage origin.[ 10 16 18 ] Zhang et al.[ 25 ] proposed a modified classification for the intracranial dissecting aneurysms, where they are classified into five subtypes (Ia, Ib, II, III, and IV). Type I is divided into two subgroups, but both of them present a thrombus inside the aneurysm; the MRI findings indicate an acute intramural hematoma. This kind of aneurysm is usually accompanied by arteriostenosis of the artery that contains the aneurysm and usually is smaller than 10 mm. Type Ia is represented by a classic ruptured dissecting aneurysms. Type Ib is the classic unruptured dissecting aneurysms. Type II represents those aneurysms that present a segmental ectasia, without thrombus formation. Type III is a chronic dissection that leads to a lateral and longitudinal extension through the artery, forming a dolichoectatic dissecting aneurysm, represented by the ectasia, elongation, and tortuosity of the vessel. Type IV is characterized by a large mural bleeding ectasia, formed by chronic intramural bleeding; this type of aneurysm is usually bigger than 10 mm.[ 25 ]
Some reports have described recurrence of aneurysmal dilatation or rebleeding after endovascular trapping. One crucial factor to this is that preoperative imaging exams cannot define the exact size and range of a fusiform aneurysm or the location of the entry point when the dissection of the intimal IEM is present.[ 17 ] Therefore, the diagnostic imaging cannot precisely characterize the entry point of the dissection, what is crucial to define a surgical treatment plan.[ 16 17 ]
The different aspects of the dissecting aneurysms cannot be divided; they are all interconnected and may dynamically transform into each other depending on the efficacy of the repairing mechanism of the intramural thrombus. Because of the hemodynamic dynamicity of the pathological mechanisms, the imaging presentation of spontaneous dissecting aneurysms is known to be variable and instable.[ 16 ]
The patient can present symptoms and signs of occlusion, arterial rupture, or mass effect.[ 4 16 18 ] Fusiform aneurysms present more often with ischemic stroke or mass effect.[ 21 ]
The best therapeutic choice for treatment of dissecting aneurysms, mainly the ones at the vertebrobasilar system, is controversial because of their morphology and the involvement of the parent vessel.[ 1 11 ] Ruptured vertebral-basilar dissecting aneurysms (VBDAs) are associated with a poor natural history with high rates of rebleed, stroke, and death when left untreated. Unruptured VBDA when not associated with stroke or mass effect usually present an excellent clinical course; however, they have a tendency to rupture and stroke when symptomatic. Both surgical and endovascular treatment (EVT) have proved to be successful when it comes to treating fusiform aneurysms. On account of its lower rates of treatment-related morbidity as well as their efficacy, EVTs have emerged as the treatment of choice.[ 4 8 24 ] However, some endovascular approaches to the treatment of VBDA exist. The EVT can be reconstructive, like stent placement, flow diversion (FD), and stent-assisted coiling or deconstructive techniques such as parent artery occlusion and trapping of the aneurysm.[ 22 ] A deconstructive treatment sacrifices the parent artery, such as a proximal occlusion of the parent artery that is performed by using balloons or coils at the segment proximal to the VBDA or the internal coil trapping that is a coil embolization of the parent artery at the dissected portion. In contrast, reconstructive treatments preserve the parent artery and can use one to three overlapping stents, alone or with coiling.[ 4 ] The FD is an endovascular technique developed for the treatment of intracranial aneurysms. It is based on the modification of blood flow induced by a stent, which over time will be covered by neointimal endothelium on such a way that the stent will be incorporated into the parent vessel. The stent will change the blood flow of an aneurysm inflow zone, in and around that area; this flux alteration will lead to a gradual intra-aneurysmal thrombosis and posteriorly atrophy and the flow into the parent vessel and perforating branches will be preserved. Flow-diversion technique can be a good option for the treatment of large, giant, and wide-necked; so, fusiform intracranial aneurysms have an anatomic feature for this technique. The FD relies on the strategy of placing the stent across the aneurysm neck or across the diseased segment of a vessel, that's why it can be a good option to treat a fusiform aneurysm.[ 1 3 8 15 ] An aneurysm becomes occluded from the circulation, repairing the diseased parent vessel segment, allowing the restoring of the normal flux in the zone.[ 1 7 15 ] Following deployment of the FDS, the aneurysm begins to thrombose and subsequently shrinks and collapses around the device. Over the ensuing 6–12 months, as endothelialization progresses and aneurysmal thrombosis continues, the parent vessel is reconstructed with eventual aneurysmal occlusion.[ 1 15 ] Recently, FD has been defended as the treatment of choice for anterior circulation fusiform aneurysms, but the technique results in the posterior circulation are variable and not well known.[ 11 15 ] However, fusiform and posterior circulation aneurysms are a risk factor for complications caused by the FD and show higher rates of morbidity and mortality.[ 15 ]
The involvement of the posterior inferior cerebellar artery (PICA) origin is an important factor to be analyzed after choosing a treatment. When internal coil trapping is used for VBDA that involved the PICA origin, the dissected segment, including the ruptured portion that either had a daughter sac or is the most notably dilated, is embolized, but the PICA origin needs to be preserved. When treating VBDs involving the PICA origin with stents and coils, a small portion of a dissecting aneurysm from which the PICA originated didn't suffer coil embolization. That is, when the PICA origin is involved, the dissecting aneurysm is left partially open; through internal coil trapping or stenting with coiling, or entirely open; through proximal occlusion or stenting alone, to preserve blood flow to the PICA. When the PICA origin is involved, neither deconstructive nor reconstructive EVTs are effectively able to completely obliterate the dissected segment, because the flux to the PICA has to be preserved.[ 4 ]
Management of intracranial basilar dissecting aneurysms has been controversial and challenging and both surgical and conservative treatments usually have a bad prognosis.[ 13 14 ] There are many endovascular techniques to treat fusiform aneurysms in basilar artery (BA). The stent-assisted coil technique is a choice to treat the lesion. However, there is a possibility of coil protrusion into parent artery; the vasculopathy is often around the parent artery in fusiform aneurysms. Deconstructive endovascular procedures as the occlusion of the vertebral and basilar arteries are an easier option, but the effect of occlusion of dominant or bilateral vertebral arteries is in doubt because persistent countercurrent flow impinges the fragile ruptured fragile wall of the lesion. Only patients with sufficient collateral arteries can tolerate a BA occlusion. A flow diverter (FD) device may be an effective option. However, the safety of the procedure is not well accepted once that the closure of the perforating branches stemming from BA always occurs. Telescopic stents without coiling technique are another option; but the size of the lesion may influence the outcome, once that stents can become instable when the lesion is large or long. Coils may help with the stents stability. Besides that, the two procedures can show better results in unruptured cases. Balloon remodeling also known as “balloon-then-stent” technique is another option. Another option is to place coils in aneurysms first and then deliver a stent around the fusiform aneurysm to compress them. The aim of this technique is to prevent coil loops herniation. The stent has two important functions; it reconstructs the vessel lumen while compressing the coils “packing them,” even when the coils are around an aneurysm. Besides that, without the presence of the stent, the coils exert a low pressure on the aneurismal walls at the initial phase. The pressure on the vessel wall, which already is affected, has to be relatively low to make the risk of rupture decrease. This technique is mainly used to prevent parent artery compromise caused by accidental prolapse or unstable coil loops during embolization of aneurysm.[ 24 ]
The authors performed a literature review using PubMed, limited to the studies published in English from 2003 to 2017. Intracranial, fusiform, aneurysms, treatment, and basilar were the key words for the article. A total of 50 articles were raised, but only 25 had the data that interested to the article construction. The data from 1556 patients that presented at least 1 intracranial fusiform aneurysm were analyzed. The patient's features; the clinical presentation; the aneurysm pathogenesis, localization, and morphology; and the treatment outcome for different treatments were observed. The studies used determined the outcome of the patient using the modified Rankin scale, and the severity of the SAH using the Hunt and Hess score. Due to retrospective design of this literature review, we did not apply for ethics committee approval.
Patient's features
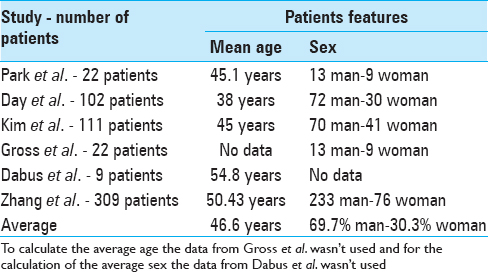
Park et al.,[ 18 ] after analyzing 22 patients that presented a fusiform aneurysm, found that 59% are men and 41% are women; 60% of them were younger than 50 years.[ 18 ]
Day et al.[ 6 ] group analyzed 102 fusiform aneurysms on the MCA. The mean age at symptom onset was 38 years. Seventy-one percent of the patients were man.[ 6 ]
In the study by Kim et al.,[ 4 ]111 patients underwent EVT for 119 VBDAs. Sixty-three percent are men and 37% are women. The average age was 45 years.[ 4 ]
Gross et al.[ 9 ] group analyzed the records of children undergoing cerebral or spinal angiography. Of 763 pediatric cases, 33 were of children harboring cerebral aneurysms. Sixty-seventy percent harbored fusiform/dissecting aneurysms. Among them, 59% were male. Patients aged 0–10 years were significantly more likely to present nonsaccular, dissecting/fusiform aneurysms than the saccular pattern.[ 9 ]
Dabus et al.[ 5 ] group analyzed nine patients who underwent EVT of fusiform intracranial vertebral artery (VA) aneurysms using reconstructive techniques. The average age of the patients was 54.8 years.[ 5 ]
Zhang et al.[ 25 ] analyzed a total of 309 patients that presented with an intracranial dissecting aneurysm. The average age was 50.43. About 75.4% of the patients were male and 24.6% female.[ 25 ]
The patients age and sex were compared and presented in Table 1 .
Table 1
Patients Features: Mean age and sex average
Clinical presentation
Park et al.[ 18 ] found that 36% of the patients analyzed presented SAH, 9% intracerebral hemorrhage, 18% neurological deficits due to ischemia, 23% dizziness, and 4.5% suffered cranial nerve deficit from mass effect.[ 18 ]
The study made by Day et al.[ 6 ] showed that 80% of the patients presented nonhemorrhagic symptoms or no symptoms at all. Clinical presentation was most often prompted by mass effect or thromboembolic stroke.[ 6 ]
Schnell et al.[ 19 ] presented three patients with fusiform aneurysms, one of them presented a stroke with expressive aphasia and ataxia; one had diplopia and the other didn't have any symptom.[ 19 ]
In the study made by Bhogal et al.,[ 2 ] an MRI was performed in 53 patients and among the patients who presented a fusiform aneurysm, 41.6% suffered mass effect, 16.6% had T1 hyperintensity, 29% had thrombus inside an aneurysm, and 33.3% already had established infarcts in the posterior circulation territory. These results demonstrate that the ischemic stroke risk is high and higher than the risk of bleeding and the rate of infarction related to the aneurysms is high too.[ 2 ]
A report of three cases of giant fusiform aneurysms made by Horie et al.[ 10 ] in the MCA shows hemorrhages of different origins. Sixty-six percent of the patients suffered a SAH and 33% had an intramural hemorrhage.[ 10 ]
Kim et al.[ 4 ] found that 61.3% VBDAs presented with SAH (ruptured aneurysms).[ 4 ]
For the 67 patients with ruptured fusiform aneurysms reported by Zhang et al.,[ 25 ] the severity of clinical manifestations was rated using the Hunt and Hess scale. Fifty two of them were a Hunt and Hess grade I, nine were grade II, four were grade III, and one was grade IV.[ 25 ]
Aneurysm morphology
Schnell et al.[ 19 ] group used a 4D-flow MRI for the comprehensive in-vivo analysis of hemodynamics and its relationship to size and morphology of different intracranial aneurysms in 18 patients, and 19 aneurysms were analyzed. Sixteen percent of these aneurysms had a fusiform morphology and all of them were large/giant. In the 3D blood flow visualization, the fusiform aneurysms demonstrated slow flow with less defined flow. Sixty-six percent of them exhibited slow flow channels across the aneurysm center and along the wall, whereas 33% barely expressed a visible flow direction with very slow swirling flow. This type of aneurysm exhibited slower and more unidirectional flow compared to saccular ones.[ 19 ]
Bhogal et al.[ 2 ] group identified 56 patients with 58 nonsaccular aneurysms of the posterior circulation. About 41% of them had a fusiform aspect.[ 2 ]
A retrospective record review was performed by Takemoto et al.[ 23 ] of patients aged <20 years treated with endovascular methods for intracranial aneurysms; 35 aneurysms were analyzed as total. About 48.5% were fusiform aneurysms.[ 23 ]
Aneurysm localization
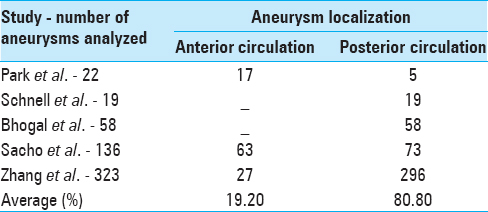
Park et al.[ 18 ] showed that 77% of the aneurysms were located in the anterior circulation; 23% were at the posterior circulation. Fifty-four percent were at the MCA (17% M1, 25% MCA bifurcation, 33% M2, 25% M3); 14% at the ICA; 9% at the ACA; 4.5% at the posterior cerebral artery (PCA); 9% at the VA, and 9% at the posterior inferior cerebellar artery (PICA).[ 18 ]
Day et al.[ 6 ] demonstrated that most of the fusiform aneurysms analyzed were originated from the M1 or M2 segments.[ 6 ]
Among the fusiform aneurysms of Schnell et al.[ 19 ] study, 100% involved the basilar artery and 33% of them also involved the VA.[ 19 ]
Among the fusiform aneurysms analyzed by Bhogal et al.,[ 2 ] 71% were localized in the VA, 12.5% were at basilar artery, 12.5% were at the vertebrobasilar transition, and 4% were at the basilar–PCA transition.[ 2 ]
A total of 121 patients with 138 unruptured fusiform intradural aneurysms were analyzed by Sacho et al.[ 20 ] Excluding 2 aneurysms that had diffuse presentation and analyzing the other 136 aneurysms, 23.5% of them were at ICA, 6% at the ACA, 17% at the MCA, 18% at the VA, 5% at the PICA, 18% at the BA, and 8% at the PCA. Looking at this findings, it's possible to see a small prevalence of fusiform aneurysms at the posterior circulation, mainly at the VA and the importance of the ICA and the MCA when it comes to anterior circulation fusiform aneurysms.[ 20 ] The aneurysms were divided into two groups: the atherosclerotics and the nonatherosclerotics. Patients with atherosclerotic aneurysms were more likely to have posterior circulation aneurysms and patients with nonatherosclerotics aneurysms were more likely to present anterior circulation aneurysms.[ 20 ]
Among the 323 aneurysms studied by Zhang et al.,[ 25 ]8.3% of them were at the anterior circulation and 91.7% were at the posterior circulation.[ 25 ]
The aneurysms localization is divided into anterior and posterior circulation in Table 2 .
Table 2
Fusiform aneurysms localization divided into anterior and posterior circulation
Cause and pathogenesis
Park et al.[ 18 ] could demonstrate that 73% of the fusiform aneurysms were caused by vessel dissection, 18% were caused by atherosclerosis, and 9% by a collagen disease or unknown factor. Arterial dissection with intramural hemorrhage between the intima and media producing focal narrowing of vessel was present in 18% of the cases. Arterial dissection with rupture producing bleeding into the brain or subarachnoid space was noted in 27%. Rupture of dissection into the arterial lumen produces a distal embolization in 9% and the progressive enlargement of an intramural clot leads to vessel occlusion in 4.5%. Progress enlargement of dissection both laterally and longitudinally was noted in 9% and serpentine channel within the dissected thrombotic aneurysm was noted in 4.5%.[ 18 ]
The aneurysms showed by Horie et al.[ 10 ] presented hemorrhages of different origins: one of them was a classic rupture type; the other was a dissection type; and an atherosclerosis-related thrombosis type. The authors proposed that giant fusiform aneurysms in the MCA are characterized by weaknesses in the internal elastic lamina with intimal thickening.[ 10 ]
Day et al.[ 6 ] found that 12% of the patients had the MCA lumen stenosed or occluded; focal dilatation in 56% and 32% had a serpentine aspect.[ 6 ]
Using his classification, Zhang et al.[ 25 ] classified the 323 aneurysms analyzed. About 20.74% of them were type Ia, 60.37% type Ib, 7.12% type II, 4.53% type III, and 8.09% type IV.[ 25 ]
Treatment outcome
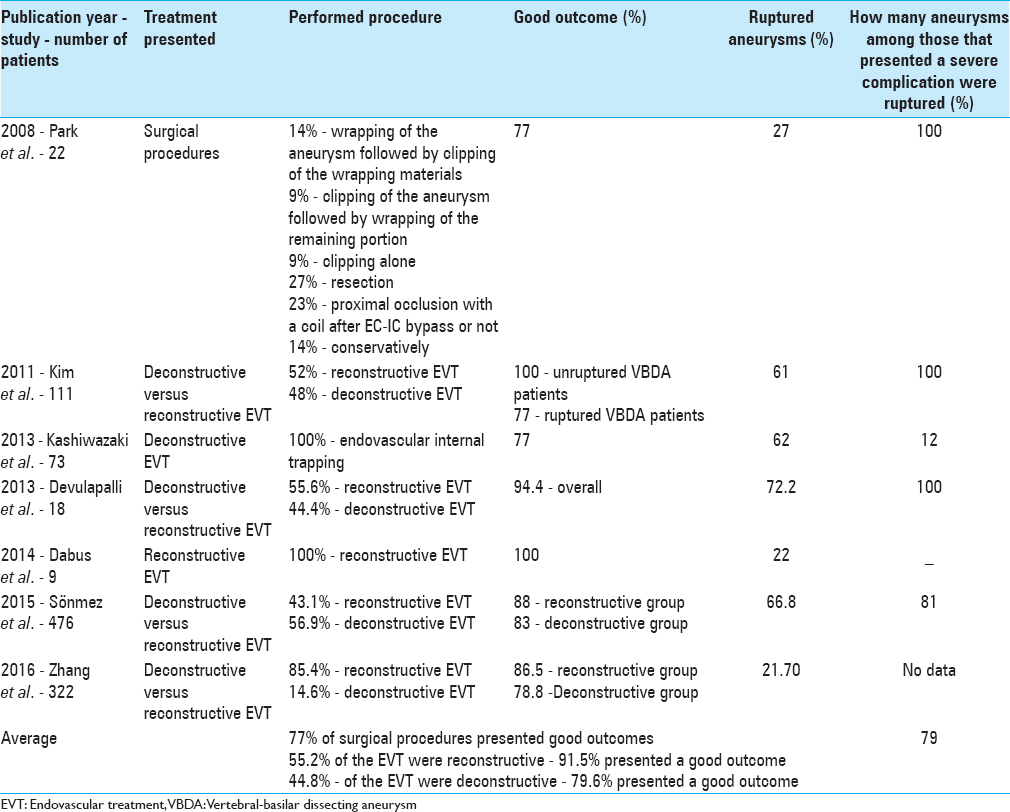
In the study made by Park et al.,[ 18 ]14% of the patients were treated with wrapping of an aneurysm followed by clipping of the wrapping materials, 9% with clipping of an aneurysm followed by wrapping of the remaining portion, 9% with clipping alone, 27% with resection, 23% by proximal occlusion with a coil after extracranial–intracranial (EC–IC) bypass or not, and 4.5% with EC–IC bypass only, and 14% of the aneurysms were treated conservatively.[ 18 ] About 77% of the patients recovered completely after treatment. Some neurological deficits developed in 14% of the patients, but they returned to full normal activities ultimately. Severe neurological deficit occurred in 4.5% of the patients caused by vasospasm during treatment of a ruptured aneurysm and the other 4.5% died because of rebleeding from a rupture of the clipped aneurysm, causing an intracerebral hemorrhage.[ 18 ]
This report made by Ota et al.[ 17 ] describes three cases in which there was a discrepancy between preoperative radiographic data and postoperative pathological findings in patients undergoing direct trapping with or without revascularization. In these cases, irregular fusiform or aneurysmal dilatation resulted from the presence of the pseudolumen below the adventitia, while segmental stenosis resulted from decreased lumen and dissection at the media. However, the data do not show the exact length of the dissecting aneurysm or the entry point. Some reports suggested that the complete occlusion of the entry point is a crucial point for a radical treatment, but the misjudgment of the dissection size, range, and entry point results in an increased risk of recurrence and rebleeding. The current diagnostic imaging cannot exactly identify the entry point and dissecting range, so open surgery is required, to have a precise determination of these parameters and also to identify small perforating branches.[ 17 ]
Devulapalli et al.[ 7 ] group analyzed 18 patients who underwent an EVT of intracranial fusiform aneurysms. About 72.2% of them presented with SAH. Technical success was achieved in 94.4%, with 55.6% undergoing reconstructive EVT and 44.4% undergoing deconstructive EVT. For patients with SAH, favorable clinical outcomes were achieved in 69.2%, with 50.0% undergoing reconstructive EVT and 85.7% undergoing deconstructive EVT. Among patients with ruptured aneurysms, only Hunt and Hess grade ≥3 was associated with an unfavorable clinical outcome. Demonstrating that the size of the hemorrhage is a predictor for bad prognose and the reconstructive EVT showed worse results, once that 75% of the patients with an unfavorable clinical outcome underwent the reconstructive method.[ 7 ]
The review made by Sönmez et al.[ 22 ] included 476 patients. About 66.8% of them presented with ruptured VBDA, and 33.2% presented with unruptured VBDA. About 43.1% were treated with reconstructive techniques, and 56.9% were treated with deconstructive techniques. Considering all patients, immediate occlusion rates were 75% and long-term occlusion rates were 87%. Angiographic recurrence rates were 7% with a retreatment rate of 3%. Perioperative morbidity was 12%. Patients with ruptured VBDA made up a majority of patients with VBDA with perioperative mortality of 11%. The overall rebleed rate for patients with ruptured VBDA was 9%.[ 22 ] Patients with ruptured VBDA treated with deconstructive techniques had higher rates of complete occlusion on immediate posttreatment angiography than those treated with reconstructive techniques (94% vs. 43%). The same was valid for long-term posttreatment angiography (95% vs. 83%). Perioperative morbidity rates were similar in the reconstructive group compared with the deconstructive group (7% vs. 14%). Perioperative mortality was 13% in the deconstructive group versus 7.0% in the reconstructive group. Long-term good clinical outcome rates were similar between the reconstructive and deconstructive groups (88% vs. 83.0%). Rebleeding rates were similar between the deconstructive and reconstructive techniques (9% vs. 7%).[ 20 ] Patients with unruptured VBDA treated with deconstructive techniques had higher rates of complete occlusion on immediate posttreatment angiography than those treated with reconstructive techniques (94% vs. 57%). The same was valid for long-term posttreatment angiography (97% vs. 68%). Perioperative morbidity rates were similar in the reconstructive group compared with the deconstructive group (7% vs. 7%). Perioperative mortality in the deconstructive group and the reconstructive group was 4% versus 5%. Long-term good clinical outcome rates were similar between the reconstructive and deconstructive groups (94% vs. 93.0%),[ 13 ] demonstrating that both deconstructive and reconstructive techniques can effectively treat ruptured and unruptured VBDA. Besides that, deconstructive techniques presented higher rates of complete angiographic occlusion and the reconstructive techniques periprocedural morbidity rates were lower. Both showed good long-term neurologic outcome and both rates of recurrence and retreatment were low. This suggests that reconstructive techniques are as effective as deconstructive endovascular techniques and safer, especially in patients who lack sufficient collateral circulation. The higher risk of neurologic complications in patients who undergo a deconstructive technique is due to ischemia resulting from sacrifice of the parent vessel. Ischemic complications at most result from the occlusion and ischemia of perforating arteries and the anterior spinal artery.[ 22 ]
Dabus et al.[ 5 ] found that 22% (2) of the patients analyzed presented with SAH (Hunt and Hess grades 2 and 3). But despite that all the patients demonstrated good outcomes after the EVT using reconstructive techniques.[ 5 ]
Kashiwazaki et al.[ 12 ] group analyzed a total of 73 patients that were treated for VA dissection (VAD) by endovascular internal trapping (deconstructive technique). Forty-five patients had ruptured VADs, and 28 had unruptured VADs. Four percent of the patients with ruptured VADs had a recurrence. Cranial nerve paresis was observed in 8.21% of them, spinal cord infarction in 2.74%, and a perforating artery ischemia was diagnosed in 9.59% patients. These findings have proven that endovascular internal trapping is a stable and durable treatment for closure of VADs. Endovascular internal trapping for VAD is a therapy with a satisfactory long-term outcome.[ 12 ]
Kim et al.[ 4 ] analyzed 62 VBDAs that were treated by a reconstructive method, 35 by using 1 to 3 overlapping stents alone, 12 using a single stent with coiling, and 15 using 2 or 3 overlapping stents with coiling. The remaining 57 VBDAs were treated by a deconstructive method, 40 by using internal coil trapping, and 17 using proximal occlusion. Clinical outcomes were favorable in all unruptured VBDA patients and in 77% (56 of 73) ruptured VBDA patients. About 23% (17) of the ruptured VBDA patients had unfavorable outcomes. About 12% (9) of the ruptured VBDA patients died, and 55% (5) of the deaths were due to rebleeding, showing that the most important point to predict a good procedure outcome is to determine if an aneurysm is ruptured, because this group represents the worse outcome.[ 4 ] Posttreatment recurrence was diagnosed when rebleeding occurred or when angiographic recurrence was confirmed. Angiographic recurrence after deconstructive treatment was defined as the presence of an enlarged dissecting aneurysm, with or without recanalization of the parent artery. After reconstructive treatment, angiographic recurrence was defined as a substantial increase in the contrast medium-filled portion of the dissecting aneurysm compared with a control angiogram taken immediately after treatment.[ 4 ]
The study made by Sacho et al.[ 20 ] demonstrates that the risk of rupture or adverse clinical events is higher in patients with fusiform aneurysms associated with intracranial atherosclerosis during the first 3 years after diagnosis. The risks of aneurysmal progression seem to be low for the majority of the patients with fusiform aneurysms not associated with intracranial atherosclerosis.[ 20 ]
Among the aneurysms studied by Zhang et al.,[ 25 ] 47 were treated by a deconstructive technique (the internal trapping) and 275 were treated by reconstructive methods (215 by stent-assisted coiling and 60 by sole stenting). In the deconstructive group, 2.1% of cases suffered an intraoperative complication (1 case of aneurysm rupture); 19.1% cases suffered postoperative complications (5 ischemic related, 3 compression related, and 1 complication by other causes). In the reconstructive group, 2.5% presented intraoperative complication (stent thrombosis in 3, aneurysm rupture in 1, stent forward migration in 1, blood flow retardation in a branch artery in 1, and iatrogenic dissection in 1); 11% suffered a postoperative complication (7 hemorrhagic related, 12 ischemic related, 7 compression related, and 4 complications by other causes). About 28 of the patients treated by a deconstructive method realized a radiographic follow-up and none of them presented recurrence of the aneurysm. A total of 246 of the patients treated by a reconstructive technique realized the radiographic follow-up and 9.7% presented recurrence of the aneurysm. The type I of the Zhang et al.[ 23 ] classification represents most of the recurrence and complicated cases, but statistically the rates are not the highest. The type III and IV present the highest rates of unfavorable outcomes.[ 25 ]
The year of the mentioned studies publication, the number of patients analyzed, the proposed treatment, the percentage of cases with good outcomes, the performed procedure, the percentage of patients that presented with ruptured fusiform aneurysms, the complications rates, and the percentage of the severely complicated fusiform aneurysms that were ruptured are presented at Table 3 .
Table 3
The year of the mentioned studies publication, the number of patients analyzed, the proposed treatment, the percentage of cases with good outcomes, the performed procedure, the percentage of patients that presented with ruptured aneurysms, the complications rates, and the percentage of the severely complicated aneurysms that were ruptured are presented
Basilar treatment
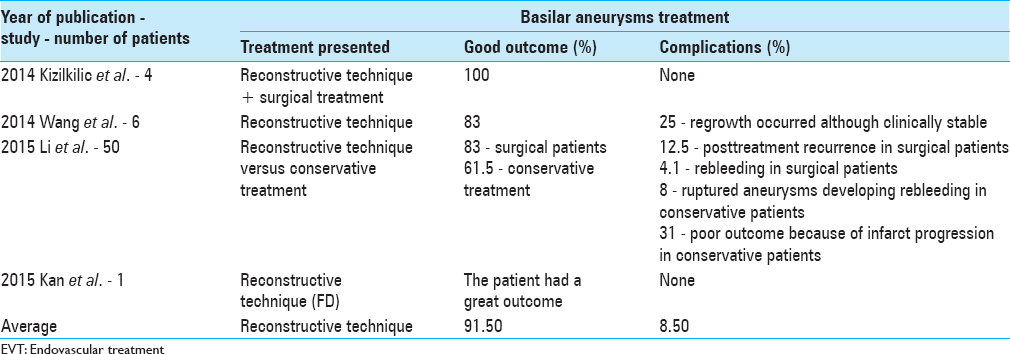
Kizilkilic et al.[ 13 ] presented the combined treatment of fusiform BA aneurysms, consisting of a surgical posterior fossa decompressive craniectomy and ventriculoperitoneal shunt operation at the same sitting, before the endovascular procedure with telescopic stenting of the aneurysmatic vessel segment in four cases. The treatment consists in a surgical procedure consisting of ventriculoperitoneal shunt placement for hydrocephalus and an occipital bone craniectomy and C1 vertebrae posterior laminectomy to decompress the posterior fossa in the same session. After surgery, the patients were loaded with acetylsalicylic acid and clopidogrel, and then, the EVT was performed. All the procedures were performed successfully without technical difficulty. The results were good. All the patients tolerated the procedures well and all cases showed remodeling with the overlapping stent technique. The study shows that this technique is a safer endovascular approach to treat symptomatic fusiform BA aneurysms by protecting patients from the hemorrhagic complications of anticoagulant therapy and thrombotic complications due to the interruption of anticoagulant therapy, at the same time treating the hydrocephalus and compression by surgical means.[ 13 ]
In this case reported by Kan et al.,[ 11 ] a symptomatic 16-year-old boy underwent a surpass FD to the treatment of a giant fusiform upper basilar trunk aneurysm. The MRI before the treatment demonstrated multiple brainstem and cerebellar infarcts and a partially thrombosed upper basilar trunk aneurysm with mass effect. The patient was pretreated with dual antiplatelet therapy, consisting of 325 mg of aspirin daily and 75 mg of clopidogrel daily for 5 days after the procedure. The patient was treated with daily dual antiplatelet therapy consisting of aspirin and clopidogrel, at the same dosage of the pretreatment, for 6 months after the procedure. Clopidogrel was then discontinued, and he remained on an aspirin regimen, 81 mg daily, which will be continued throughout his life. The results of FD in the treatment of posterior circulation aneurysms have uniformly been worse compared with anterior circulation aneurysms. Analyzing this case reported by Kan et al.,[ 11 ] it can be seen that the surpass FD has several potential advantages in the treatment of long fusiform BA aneurysms. Considering that the patient, who presented with diplopia, ataxia, dysarthria, dysphagia, and right hemiparesis, after 6 months had resolved the diplopia and dysphagia, he only had residual dysarthria and ataxia and his right-sided strength was normal; the FD was a great treatment. The surpass FD is available in longer lengths, thereby potentially allowing the use of a single device and minimizing the coverage of perforators branches and there is less device foreshortening, especially in fusiform aneurysms where the device will expand to nominal diameters with maximal foreshortening. With less foreshortening and more predictable length of the device, it reduces the need of telescoping multiple devices, thus enhancing the chance of perforator preservation.[ 11 ]
Fifty patients with basilar dissecting aneurysms were analyzed by Li et al.;[ 14 ] 24 underwent stent-assisted coiling, whereas 26 patients underwent conservative treatment. Of the patients treated with stent-assisted coiling, 83% had a favorable outcome, posttreatment recurrence occurred in 12.5% of the patients, and one had rebleeding. Of the patients treated with conservative therapy (observation or anticoagulation), 38.5% of the patients had an unfavorable outcome, 8% of the patients with ruptured aneurysms developed rebleeding, and 31% had poor outcome due to infarct progression. The stent-assisted coiling group presented better outcome compared to the conservatively treated group. Stent placement and initial complete occlusion were the favorable factors in patients with BA dissecting aneurysm.[ 14 ]
Six patients with fusiform aneurysms in the BA treated by stenting following coiling technique were analyzed by Wang et al.[ 24 ] They had a total of seven aneurysms in the BA. About 33% of the patients had SAH, whereas 67% had unruptured aneurysms. The patients with SAH were admitted with Hunt and Hess scale grade III and IV. Among the four patients with an unruptured aneurysm, 75% presented with progressive brainstem symptoms caused by compression from a markedly elongated BA, and 25% had symptoms of brainstem ischemia, demonstrating the importance of the ischemia and mass effect on the fusiform aneurysms. Patients were routinely given dual antiplatelet agents (75 mg clopidogrel and 100 mg aspirin) for 3 days before operation. In acute SAH phase, the patient was given a loading dose of dual antiplatelets (clopidogrel 300 mg and aspirin 300 mg) through stomach tube before procedure. The dual antiplatelet agents (75 mg clopidogrel and 100 mg aspirin) were given once a day for 1 month after the procedure. Then, 100 mg of aspirin was continued for the next 6 months. Both patients with SAH had no rehemorrhage during the observation period. The other four patients didn't present new neurological deficit and also showed an important improvement in their presenting symptoms; 75% of them demonstrated complete aneurysm occlusion with reconstructive BA patency. Regrowth occurred in 25% of them at 1 year follow-up, although clinically stable.[ 24 ]
The year of the mentioned studies publication, the number of patients analyzed, the proposed treatment for a fusiform aneurysm at the BA, the percentage of cases with good outcomes, and the complications rates are presented in Table 4 .
Table 4
The year of the mentioned studies publication, the number of patients analyzed, the proposed treatment for a fusiform aneurysm at the basilar artery the percentage of cases with good outcomes, and the complications rates are presented
Looking for the patient that has an intracranial fusiform aneurysm features, most of them are men;[ 4 17 18 ] most of them are younger than 50 years old[ 4 5 6 18 ], and the most affected population is the pediatric patients.[ 4 9 11 16 23 ]
Dividing the clinical presentation as ischemic/mass effect and hemorrhage symptoms, it can be seen that the majority of symptoms are caused by ischemic/mass effect;[ 2 6 18 19 21 ] aphasia, ataxia, diplopia, dizziness, cranial nerve deficit, and hemiparesis are some of the symptoms that can be seen after ischemia or mass effect.[ 18 19 ] The hemorrhage can have different origins, depending on the mechanism of formation of an aneurysm, but the most common is the SAH.[ 4 10 ] This type of hemorrhage occurs when the disruption of the vessel wall advance to the adventitia resulting in rupture and SAH.[ 17 ]
The fusiform morphology is less common when compared to the saccular pattern.[ 2 19 23 ] The fusiform aneurysms mainly are presented as a large/giant aneurysm.[ 19 ] This kind of an aneurysm causes a slow blood flow across the aneurysm center and along the vessel lumen.[ 19 ] This verification demonstrates the narrowing process of the vessel and the ischemic compound of the evolution of a fusiform aneurysm and elucidates the importance of the mass and hipoflux effect on the fusiform aneurysms, and the dissection pattern may be the explanation for the slow flow.[ 19 ]
The fusiform aneurysms is often presented in the posterior circulation, mainly in the VA followed by the BA, PICA, and PCA.[ 2 16 18 19 20 25 ] This kind of aneurysm in the anterior circulation is less frequent but some studies have been showing its importance as Park et al.[ 1 ] and Sacho et al.[ 20 ] In the anterior circulation, the most committed arteries are the MCA[ 16 18 ] and the ICA followed by the ACA[ 16 18 ]. The M2 segment of the MCA and the supraclinoid segment of the ICA are the most important sites at the anterior circulation.[ 6 16 18 ] Onofrj et al.[ 16 ] group hypothesize that the supraclinoid ICA segment represents a preferential site of dissection because a point of local weakness of the internal elastic lamina may occur after the division of the primitive ICA. The atherosclerosis seems to influence the rate of distribution from this aneurysm pattern. Patients with atherosclerotic aneurysms are more likely to have posterior circulation aneurysms and patients with nonatherosclerotics aneurysms are more likely to present anterior circulation aneurysms according to Sacho et al.[ 20 ]
The principal mechanism to form a fusiform aneurysm is the dissection of the internal wall vessel, what communicates the true lumen and the pseudolumen through a disrupted portion (entry point) of the internal elastic lamina.[ 10 17 18 ] This leads to dissection with intramural hemorrhage between the intima and media producing focal narrowing of vessel.[ 6 10 16 17 18 ] After this point, there are some possibilities to the aneurysm evolution. The arterial dissection can rupture, producing bleeding into the brain or subarachnoid space.[ 10 16 17 18 ] It can occur the rupture of dissection into the arterial lumen producing a distal embolization[ 1 5 ] and the intramural clot can expand leading to vessel occlusion.[ 6 16 18 ] A progress enlargement of dissection both laterally and longitudinally can happen and a serpentine channel forms as disease extends longitudinally, combined with varying degrees of intraluminal thrombosis.[ 6 18 ] Dividing fusiform aneurysms according to their radiographic appearance showed importance in the treatment outcome, so according to Zhang et al.[ 25 ] determine if a fusiform aneurysm is a classic dissecting aneurysm, a segmental ectasia, a dolichoectatic dissecting aneurysm or a large mural bleeding ectasia is crucial to determine the treatment and its prognosis. A dolichoectatic dissecting and a large mural bleeding ectasia like aneurysm showed worst prognosis.[ 25 ]
There are many techniques to treat a fusiform aneurysm; it can be an EVT, a surgical procedure, or a conservative treatment. Wrapping of an aneurysm followed by clipping the wrapping materials, clipping of an aneurysm followed by wrapping of the remaining portion, clipping alone, resection, proximal occlusion with a coil after a EC–IC bypass or not, and EC–IC bypass only are some surgical techniques to the aneurysm treatment.[ 18 ] Trapping with revascularization by open surgery is another good option for management of ruptured intracranial dissecting aneurysms, because endovascular trapping has the risk of branch occlusion, which can lead to cerebellar and brainstem infarction, due to the difficulty in localizing the perforating branches and the entry point.[ 17 ] Good results are presented. However, there are severe limitations in the outcome when it comes to ruptured aneurysms.[ 18 ] An important point to prepare a surgery is the lack of imaging exams that can show the exact length of the fusiform aneurysm or the precise localization of the entry point. The two cases reported by Onofrj et al.[ 16 ] show the hemodynamic dynamicity of the fusiform aneurysms and how they can change its pattern fast and cause at the same time a SAH and ischemic symptoms. The suspected point of transmural rupture was contained in the fusiform dilation in the first case and appeared just distal to the dilation in the second case, what shows that current diagnostic imaging cannot precisely characterize the entry point and dissecting length.[ 16 ] Some reports suggested that the complete occlusion of the entry point is crucial for the effective treatment, but a misjudgment on the evaluation of the dissecting size, range, and entry point results in an increased risk of recurrence and/or rebleeding and the current imaging exams cannot precisely characterize the entry point and dissecting range, so open surgery is required for a better characterization of these data and also to identify small perforating branches.[ 17 ] So, surgical procedures still have an important place in this field. The EVT can be divided into two groups, the reconstructive and the deconstructive. A deconstructive treatment sacrifices the parent artery; a proximal occlusion of the parent artery is performed by using balloons or coils at the segment proximal to the VBDA, and internal coil trapping is coil embolization of the parent artery at the dissected segment. In contrast, reconstructive treatments preserve the parent artery and use one to three overlapping stents, alone or with coiling.[ 4 ] Reconstructive techniques showed some good results even in ruptured aneurysms that is a predictor for bad prognosis. Among patients with ruptured aneurysms, only Hunt and Hess grade ≥3 was associated with an unfavorable clinical outcome.[ 5 7 ] But Devulapalli et al.[ 7 ] found better results using the deconstructive methods, once that 75% of the patients with an unfavorable clinical outcome underwent the reconstructive methods.[ 7 ] Sönmez et al.[ 22 ] also found that patients with unruptured VBDA treated with deconstructive techniques had higher rates of complete occlusion on immediate posttreatment angiography than those treated with reconstructive techniques.[ 22 ] But periprocedural morbidity rates were lower for reconstructive techniques.[ 22 ] Overall, these findings suggest that reconstructive and deconstructive techniques are both effective;[ 7 12 22 25 ] the reconstructive techniques are possibly safer than deconstructive techniques, especially in cases in which patients lack sufficient collateral circulation. Similar rates of good long-term neurologic outcome between patients treated with reconstructive and deconstructive techniques were found; however, higher rates of perioperative morbidity are seen among patients treated with deconstructive techniques. Deconstructive techniques increase the risk of neurologic complications secondary to ischemia resulting due to sacrifice of the parent vessel. Ischemic complications, at most, are the result of occlusion and ischemia of perforating arteries and the anterior spinal artery.[ 22 25 ] Zachary et al.[ 25 ] also showed that the deconstructive techniques presented lower recurrence rates of the aneurysm. The most important feature of an aneurysm to predict a bad prognose is to determine if an aneurysm is ruptured and the clinical manifestations due to the hemorrhage (Hunt and Hess).[ 4 7 12 22 25 ] The atherosclerosis seems to be a risk factor for an aneurysm rupture.[ 20 ]
The management of intracranial basilar dissecting aneurysms has been controversial and challenging and both surgical and conservative treatments usually have a bad prognosis.[ 13 14 ] Fusiform aneurysms in BA can be treated by many endovascular techniques. Combined treatment of fusiform BA aneurysms, consisting in a surgical posterior fossa decompressive craniectomy and ventriculoperitoneal shunt operation at the same time, before the telescopic stenting of the aneurysmatic BA segment in four cases presented, showed excellent results and seems to be a safer endovascular approach to treat symptomatic fusiform BA aneurysms, once it protects the patients from the hemorrhagic complications of the anticoagulant therapy and from the thrombotic complications owing to the interruption of the anticoagulant therapy, at the same time treating the hydrocephalus and compression by surgical procedures.[ 13 ] The FD is an endovascular technique developed for treatment of intracranial aneurysms, more usually used for anterior circulation aneurysms, but Kan et al.[ 11 ] used FD to treat a giant fusiform upper basilar trunk aneurysm and had a great result, and according to Briganti et al.,[ 3 ] FD is the best option to the treatment of fusiform aneurysms. This technique minimizes the coverage of perforators branches and increases their preservation.[ 11 ] Li et al.[ 14 ] compared the outcome of a reconstructive EVT and a conservative treatment. The endovascular treated group had a more favorable outcome than the conservatively treated group.[ 14 ] Li et al.[ 14 ] and Wang et al.[ 24 ] demonstrate good results using the stent-assisted coil technique and stenting following coiling technique, respectively (both are reconstructive methods).[ 14 24 ] Stenting following coiling is an alternative strategy of stent-assisted coil technique. This seems to be a feasible and practical method to remodel the parent artery and coil this type of aneurysms.[ 24 ] A critical remark is that all the patients treated for a basilar fusiform aneurysm were treated with dual antiplatelet after and before the procedure.[ 11 13 24 ] The medication dosage and period varies, depending on the treatment and the occurrence of SAH or any hemorrhage manifestation. However, there is a lack of data about the treatment of fusiform BA aneurysms. There is no systematic revision about the topic and no study reveals consistent data about the realization of deconstructive techniques on fusiform aneurysms in this location, probably because the sacrifice of the BA would lead to catastrophic consequences.
The treatment of a VBDA that involved the PICA origin is another particular case and has to be determined on a case-by-case basis, according to each patient's symptoms; hemodynamic status, the sufficiency of the collateral supply, is very important and anatomic features of the vertebrobasilar artery also are. It can be seen in particular that the involvement of the PICA origin was the only independent risk factor for recurrence after an EVT of VBDAs. The PICA origin involvement makes the dissected segment completely obliteration impracticable, neither using deconstructive nor reconstructive treatment, because the blood flow to the PICA needs to be preserved. This scenario necessarily allows continuous antegrade or retrograde blood flow through the remnant aneurysm sac to the PICA. So, the persistent flow through the unprotected remnant dissecting aneurysm toward the PICA may be the cause for the recurrence. Given the high rates of recurrence for VBDA with PICA origin involvement and the horrible outcome of rebleeding, deconstructive treatment including PICA occlusion should be considered, especially in ruptured VBDAs with PICA origin involvement. However, if the contralateral PICA is absent and the ipsilateral, anterior inferior cerebellar artery is hypoplastic, the occlusion of the affected PICA in ruptured VBDAs may cause a large cerebellar infarct. Therefore, under such anatomic conditions, an occipital artery–PICA bypass surgery may be performed.[ 4 ]
Most of the patients are men, younger than 50 years old, and the most affected population is the pediatric patients. The majority of symptoms are caused by ischemic/mass effect. The fusiform morphology is less common when compared to the saccular pattern. The fusiform aneurysms are mostly presented in the posterior circulation, mainly in the VA. The current diagnostic imaging cannot precisely characterize the entry point, dissecting length, and identify small perforating branches. So, surgical procedures still have an important place in this field. Reconstructive and deconstructive techniques are both effective; the reconstructive techniques are possibly safer than deconstructive techniques, especially in patients who lack sufficient collateral circulation. The most important feature of an aneurysm to predict a bad prognose is to determine if the aneurysm is ruptured. The reconstructive EVT accompanied by dual antiplatelet after and before the procedure showed the best results to treat the fusiform BA aneurysms. Deconstructive treatment including PICA occlusion should be considered, given the high rates of recurrence for VBDA with PICA origin involvement and the extremely bad outcome of rebleeding, especially when the VBDA is ruptured.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.