- Department of Neurosurgery, Kurume University School of Medicine, Asahi-machi, Kurume, Fukuoka, Japan.
DOI:10.25259/SNI_210_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jin Kikuchi, Yasuharu Takeuchi, Keisuke Sugi, Tetsuya Negoto, Munetake Yoshitomi, Masaru Hirohata, Motohiro Morioka. Gamma knife surgery-induced aneurysm rupture associated with tissue plasminogen activator injection: A case report and literature review. 26-Jul-2019;10:150
How to cite this URL: Jin Kikuchi, Yasuharu Takeuchi, Keisuke Sugi, Tetsuya Negoto, Munetake Yoshitomi, Masaru Hirohata, Motohiro Morioka. Gamma knife surgery-induced aneurysm rupture associated with tissue plasminogen activator injection: A case report and literature review. 26-Jul-2019;10:150. Available from: http://surgicalneurologyint.com/surgicalint-articles/9527/
Abstract
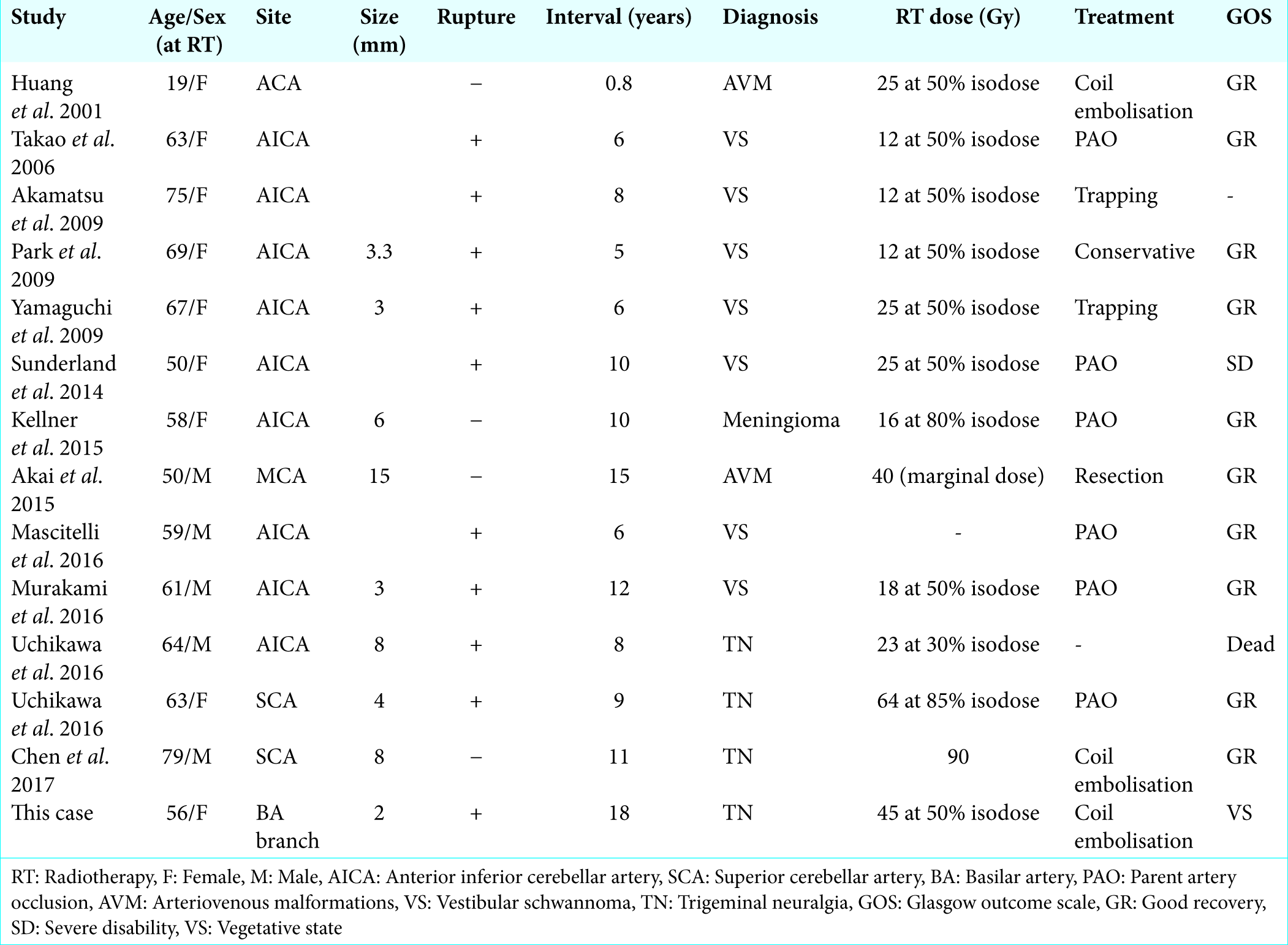
Background: Cases involving delayed development of intracranial aneurysms related to gamma knife surgery (GKS) have been recently reported. Here, we present a rare case of GKS-induced aneurysm rupture after intravenous injection of tissue plasminogen activator (t-PA) for occlusion of the middle cerebral artery (MCA). To the best of our knowledge, this is the first case in which t-PA-induced rupture of a GKS-related unruptured aneurysm.
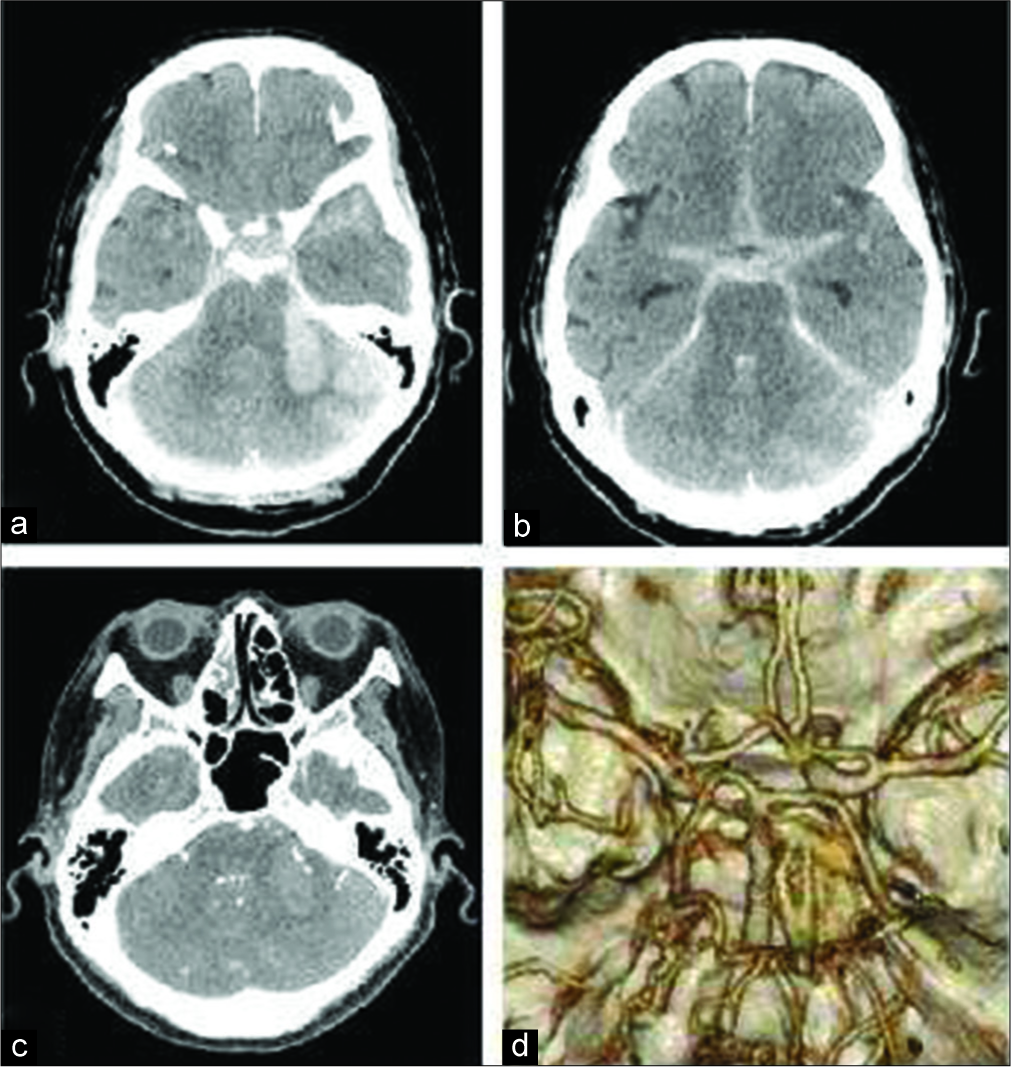
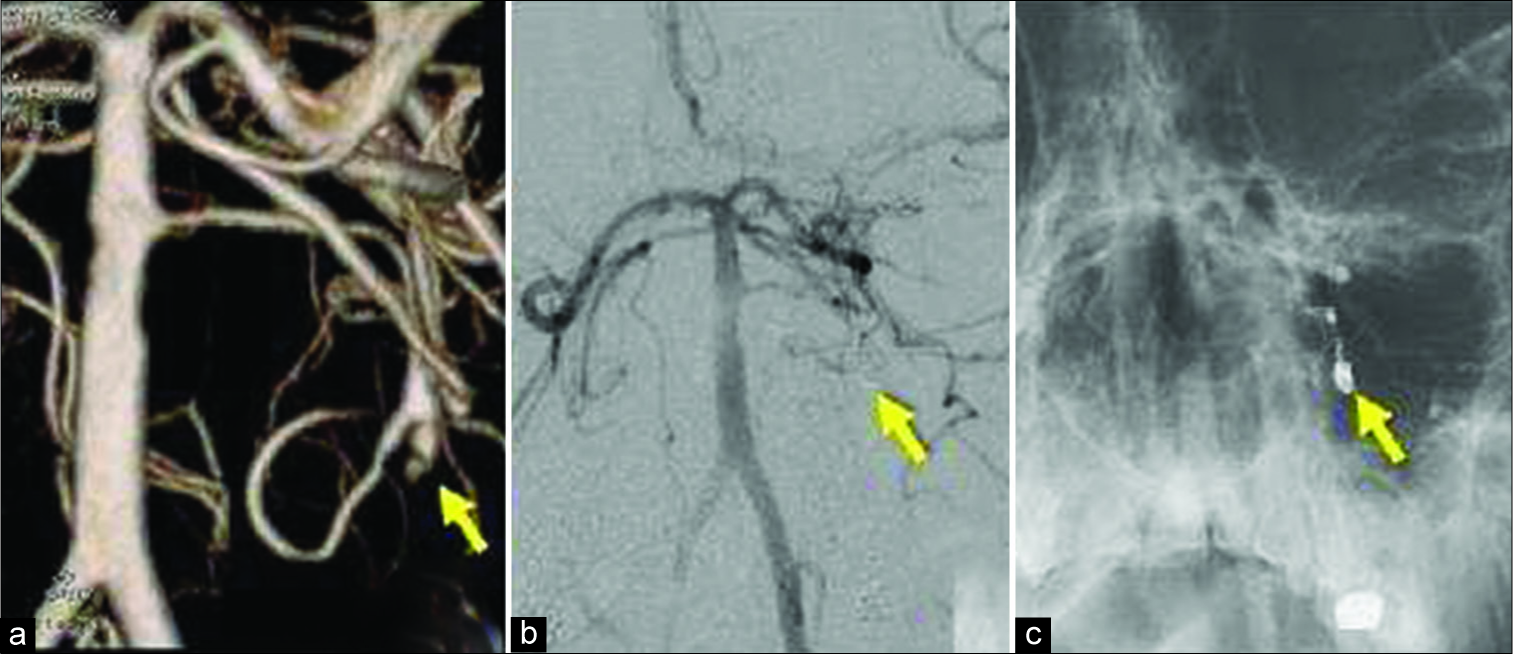
Case Description: A 56-year-old woman underwent GKS for left trigeminal neuralgia. Eighteen years later, she suddenly experienced MCA occlusion with consciousness disturbance and right hemiparesis. She received an intravenous injection of t-PA and then was transferred to our hospital. We confirmed residual thrombus, and she underwent mechanical thrombectomy successfully. A postthrombectomy brain computed tomography scan revealed subarachnoid hemorrhage with a hematoma in the left cerebellar hemisphere. Cerebral angiography revealed a small irregular-shaped aneurysm at the branching site of the left circumflex branch at the distal position of the anterior inferior cerebellar artery, which was not detected on initial imaging. Coil embolization was performed. One month after the ischemic attack, she was transferred to a rehabilitation hospital, with a modified Rankin Scale score of 5.
Conclusions: The tendency to rupture is greater for GKS-induced aneurysms than for intrinsic unruptured aneurysms, according to previous reports. When performing acute treatment for cerebral infarction in patients with a history of GKS, the presence of aneurysms should be evaluated and we should keep in mind that GKS aneurysms are very small and tend to rupture.
Keywords: Aneurysm rupture, Gamma knife surgery, Tissue plasminogen activator
INTRODUCTION
With the increase in the number of patients treated using gamma knife surgery (GKS), recent cases involving delayed development of intracranial aneurysms related to GKS have been reported.[
CASE REPORT
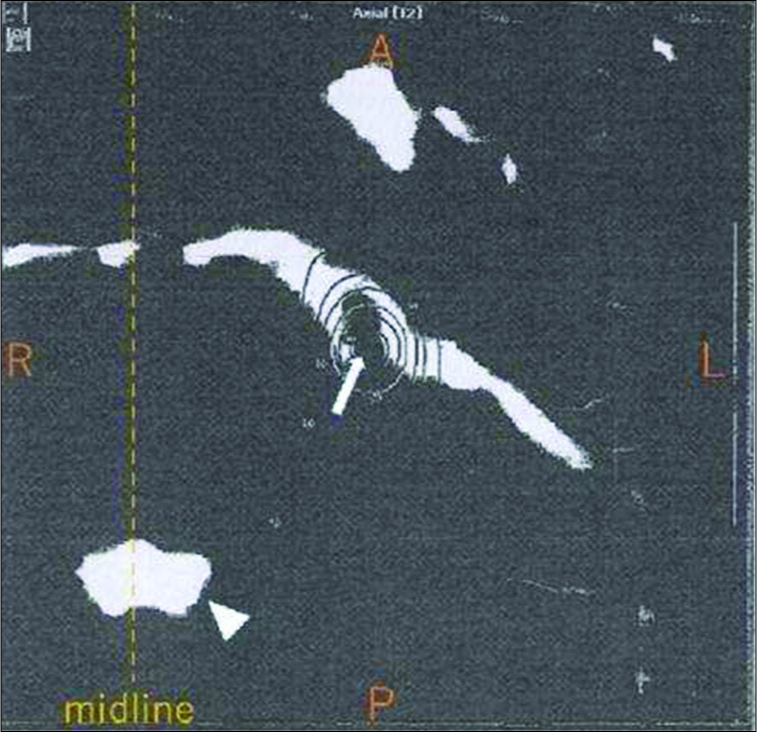
A 56-year-old woman had experienced trigeminal neuralgia (TN) and was treated using GKS. She received 45 Gy at 50% isodose at the proximal cisternal extent of the left trigeminal nerve [
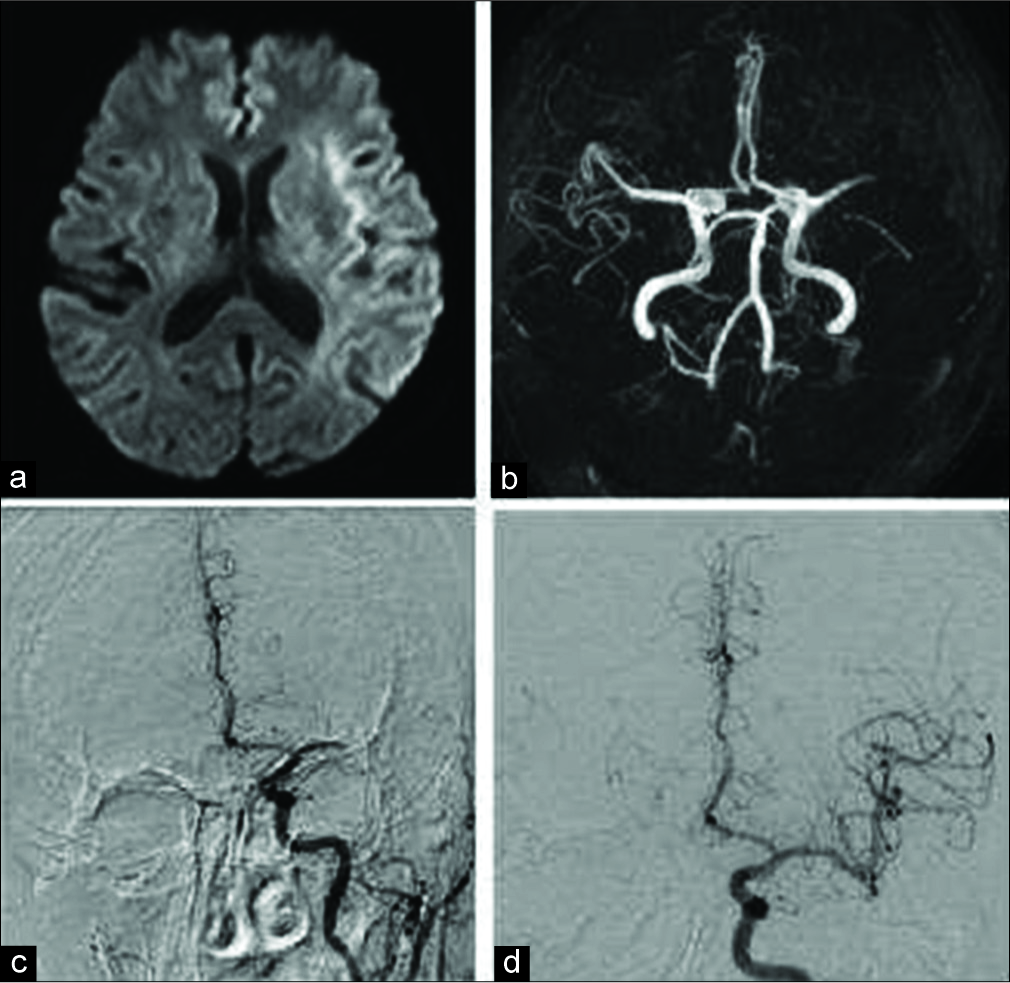
She experienced a sudden onset of consciousness disturbance and right hemiparesis (National Institutes of Health Stroke Scale score = 20 points). Brain magnetic resonance imaging was performed at the primary hospital. Diffusion-weighted images revealed a hyperintensity in the left insular cortex and a slight hyperintensity in the left MCA territory [
The patient’s family provided consent for the publication of this case report.
Figure 2:
Magnetic resonance imaging (a and b) at the first emergency department, and digital subtraction angiography before (c) and after (d) mechanical embolectomy. Diffusion-weighted image (a) shows a hyperintensity in the left insular cortex and a slight hyperintensity in the left middle cerebral artery territory. Magnetic resonance angiography (b) shows occlusion in the horizontal segment of the left middle cerebral artery. Digital subtraction angiography shows occlusion of the horizontal segment of the left middle cerebral artery (c). Complete recanalization is performed using the Solitaire FR/2 revascularization device (d). Onset to needle time: 1 h 30 min, onset to reperfusion time: 3 h 13 min, puncture to reperfusion time: 0 h 18 min.
Figure 4:
Cerebral angiography of the posterior cerebral circulation performed after computed tomography shows aneurysm-like dilatation in the peripheral portion of the left circumflex branch at the distal position of the anterior inferior cerebellar artery. Coil embolization is performed for aneurysmal dilatation. (a) Cerebral angiography shows aneurysm-like dilatation (arrow). (b and c) Postembolization angiography shows complete obliteration of the aneurysm (arrow).
DISCUSSION
GKS, which irradiate high-dose radiation to the target lesion, is one of the treatment options for intracranial arteriovenous malformations (AVMs), brain tumors, and TN. Radiation is known to be a factor in the delayed development of aneurysms. GKS-induced aneurysm formation is considered rare, but recently, the reported cases are increasing; Uchikawa et al. reported that delayed development of intracranial aneurysms following GKS was noted in 0.90% of patients.[
There are few pathological studies on GKS-induced vasculopathy. Akamatsu et al. described that the wall of the arterial aneurysm exhibited a pseudoaneurysm-like structure formed with thin collagen fibers lacking elastic fibers and a tunica media, without atherosclerotic changes.[
However, in the present case, a GKS-induced aneurysm ruptured after intravenous injection of t-PA. We consider that the rupture tendency is greater for GKS-induced aneurysms than for intrinsic unruptured aneurysms, as plasmin activated by t-PA acts on fibrin that is a component of the vessel wall of GKS-induced aneurysms. In AVMs and TN, major artery branches might be directly affected by irradiation. In TN, which is characterized by arteries directly touching the trigeminal nerve, avoidance of a high dose of radiation exposure to surrounding arteries could be technically difficult.[
In this case, we could not find the aneurysm by MRA and CTA, because it was very small and irregularly shaped. In fact, most ruptured aneurysms have a size of under 5 mm [
CONCLUSION
GKS-induced aneurysms are vulnerable, small size and have a greater tendency to rupture than intrinsic unruptured aneurysms. When performing acute treatment for cerebral infarction in patients with a history of GKS, the presence of aneurysms should be evaluated and we should keep in mind that GKS aneurysms are very small and tend to rupture.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient family has given her consent for her images and other clinical information to be reported in the journal. The patient family understands that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Keiko Nakahara for technical assistance and Editage (www.editage.jp) for English language editing.
References
1. Akai T, Torigoe K, Fukushima M, Iizuka H, Hayashi Y. De novo aneurysm formation following gamma knife surgery for arteriovenous malformation: A Case report. J Neurol Surg Rep. 2015. 76: e105-8
2. Akamatsu Y, Sugawara T, Mikawa S, Saito A, Ono S, Takayama K. Ruptured pseudoaneurysm following gamma knife surgery for a vestibular schwannoma. J Neurosurg. 2009. 110: 543-6
3. Chen JC, Chao K, Rahimian J. De novo superior cerebellar artery aneurysm following radiosurgery for trigeminal neuralgia. J Clin Neurosci. 2017. 38: 87-90
4. Chiu WT, Hong CT, Chi NF, Hu CJ, Hu HH, Chan L. The risk of intravenous thrombolysis-induced intracranial hemorrhage in Taiwanese patients with unruptured intracranial aneurysm. PLoS One. 2017. 12: e0180021-
5. Edwards NJ, Kamel H, Josephson SA. The safety of intravenous thrombolysis for ischemic stroke in patients with pre-existing cerebral aneurysms: A case series and review of the literature. Stroke. 2012. 43: 412-6
6. Huang PP, Kamiryo T, Nelson PK. De novo aneurysm formation after stereotactic radiosurgery of a residual arteriovenous malformation: Case report. AJNR Am J Neuroradiol. 2001. 22: 1346-8
7. Kellner CP, McDowell MM, Connolly ES, Sisti MB, Lavine SD. Late onset aneurysm development following radiosurgical obliteration of a cerebellopontine angle meningioma. J Neurointerv Surg. 2015. 7: e21-
8. Mascitelli JR, McNeill IT, Mocco J, Berenstein A, DeMattia J, Fifi JT. Ruptured distal AICA pseudoaneurysm presenting years after vestibular schwannoma resection and radiation. J Neurointerv Surg. 2016. 8: e19-
9. Murakami M, Kawarabuki K, Inoue Y, Ohta T. Ruptured pseudoaneurysm after gamma knife surgery for vestibular schwannoma. Neurol Med Chir (Tokyo). 2016. 56: 38-42
10. Park KY, Ahn JY, Lee JW, Chang JH, Huh SK. De novo intracranial aneurysm formation after gamma knife radiosurgery for vestibular schwannoma. J Neurosurg. 2009. 110: 540-2
11. Sunderland G, Hassan F, Bhatnagar P, Mitchell P, Jayakrishnan V, Forster D. Development of anterior inferior cerebellar artery pseudoaneurysm after gamma knife surgery for vestibular schwannoma. A case report and review of the literature. Br J Neurosurg. 2014. 28: 536-8
12. Takao T, Fukuda M, Kawaguchi T, Nishino K, Ito Y, Tanaka R. Ruptured intracranial aneurysm following gamma knife surgery for acoustic neuroma. Acta Neurochir (Wien). 2006. 148: 1317-8
13. Uchikawa H, Nishi T, Kaku Y, Goto T, Kuratsu JI, Yano S. Delayed development of aneurysms following gamma knife surgery for trigeminal neuralgia: Report of 2 cases. World Neurosurg. 2017. 99: 813-21
14. Yamaguchi S, Kato T, Takeda M, Ikeda H, Kitamura K. Ruptured distal anterior inferior cerebellar artery aneurysm following stereotactic irradiation for vestibular schwannoma: Case report. Neurol Med Chir (Tokyo). 2009. 49: 202-5