- Department of Neurosurgery, Gardens Hospital, Amman, Jordan University of Science and Technology, Irbid, Jordan.
- Department of Radiology, Division of Neurology, Jordan University of Science and Technology, Irbid, Jordan.
- Department of Neuroscience, Division of Neurology, Jordan University of Science and Technology, Irbid, Jordan.
- Department of Pathology and Laboratory Medicine, Jordan University of Science and Technology, Irbid, Jordan.
- Department of Accident and Emergency, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan.
Correspondence Address:
Bashar Abuzayed
Department of Accident and Emergency, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan.
DOI:10.25259/SNI_496_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bashar Abuzayed1, Khaled Alawneh2, Majdi Al-Qawasmeh3, Sohaib Al-Khatib4, Marwa Barukba4, Liqaa Raffee5. Ganglioglioma of optic chiasma: A case report and review of literature. 18-Nov-2020;11:392
How to cite this URL: Bashar Abuzayed1, Khaled Alawneh2, Majdi Al-Qawasmeh3, Sohaib Al-Khatib4, Marwa Barukba4, Liqaa Raffee5. Ganglioglioma of optic chiasma: A case report and review of literature. 18-Nov-2020;11:392. Available from: https://surgicalneurologyint.com/surgicalint-articles/10395/
Abstract
Background: Gangliogliomas are neoplasms containing both astrocytic and neuronal components. We present a case of gangliogliomas of the optic chiasm, which are extremely rare pathologies.
Case Description: A 16-year-old female patient referred to our clinic with gradual deterioration of vision for the age of 1 year mostly in the right eye. Ophthalmic examination confirmed reduced visual acuity with only perception of light in the left eye. Brain magnetic resonance imaging showed a solid mass lesion involving the hypothalamus and the optic chiasm, which was hypointense on T1-weighted images, hyperintense on T2-WI, and marked homogenous contrast enhancement. The patient was operated and bulging of the optic chiasm and the site of lamina terminalis was seen. Subtotal resection of the tumor was achieved. Histopathological examination revealed ganglioglioma (WHO Grade I). Follow-up of the patient was for 3 years and 8 months with stable neurologic and radiologic findings.
Conclusion: To the best of our knowledge, 20 cases, including ours, have been reported in the literature and a presurgical diagnosis of ganglioglioma is very infrequent with confused radiologically with low-grade pilocytic astrocytomas.
Keywords: Ganglioglioma, Optic chiasm, Suprasellar tumor
INTRODUCTION
First described by Courville in 1930, the term ganglioglioma was introduced to define neoplasms containing both astrocytic and neuronal components.[
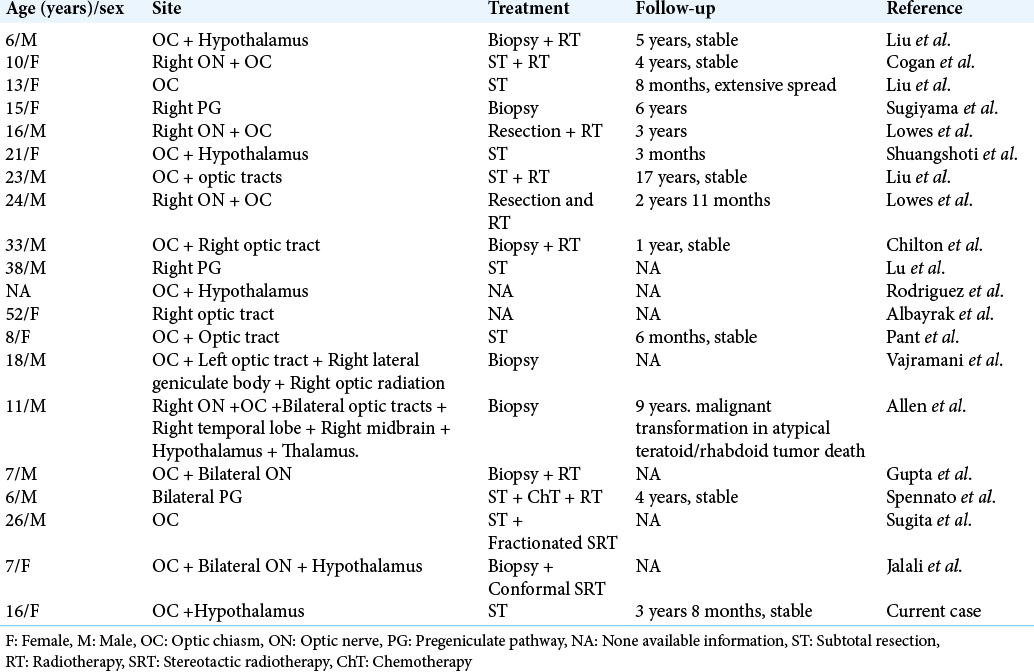
Gangliogliomas of the optic chiasm are extremely rare pathologies and intensive literature search by the authors showed only 20 cases of optic chiasm. Ganglioglioma, including the current case, has been reported so far.[
CASE REPORT
A 16-year-old female patient referred to our outpatient clinic with gradual deterioration of vision for the age of 1 year mostly in the right eye, with episodes of sharp headaches. Ophthalmic examination confirmed reduced visual acuity with only perception of light in the left eye. Neurological examination showed no other neurologic deficit. Brain magnetic resonance imaging (MRI) showed a solid mass lesion involving the hypothalamus and the optic chiasm. The lesion was hypointense on T1-weighted images, hyperintense on T2-WI, and showed marked homogenous enhancement after the administration of contrast material [
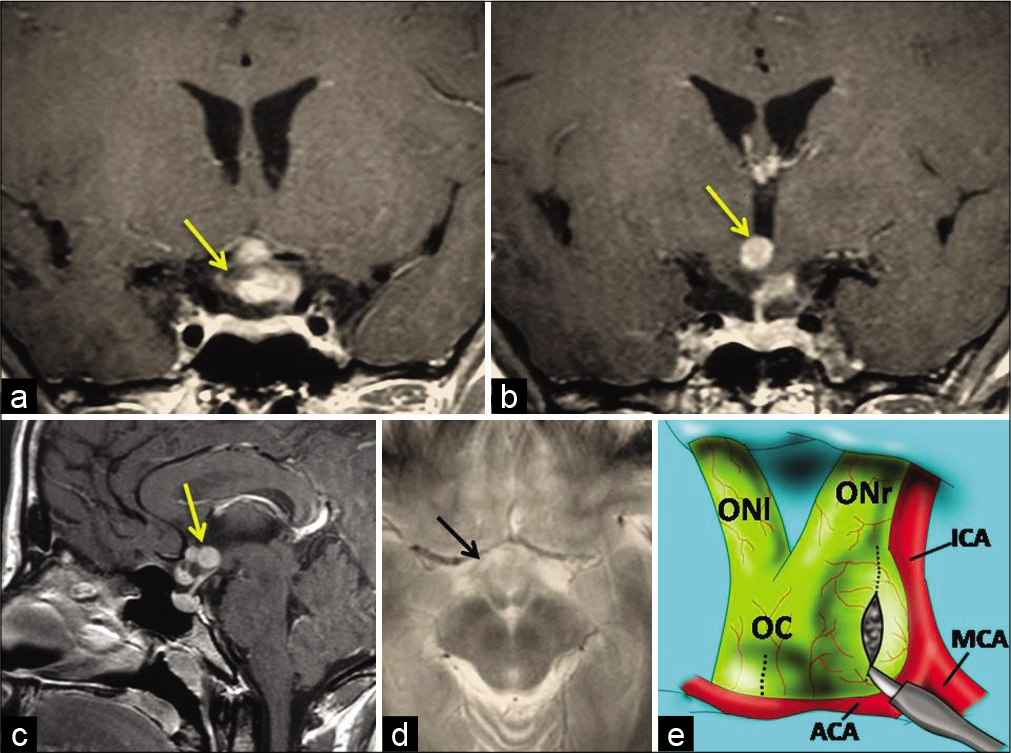
Figure 1:
Preoperative brain magnetic resonance imaging showing a mass lesion involving the optic chiasm and hypothalamus (arrows). (a and b) Coronal postcontrast, (c) sagittal postcontrast, and (d) axial T2-weighted images. (e) Schematic drawing demonstrating the enlarged optic chiasm due to tumor and the entry site through the exophytic part of the tumor in the right optic chiasm and lamina terminalis (dotted lines). OC: Optic chiasm, ONr: Right optic nerve, ONl: Left optic nerve, ICA: Internal carotid artery, MCA: Middle cerebral artery, ACA: Anterior cerebral artery.
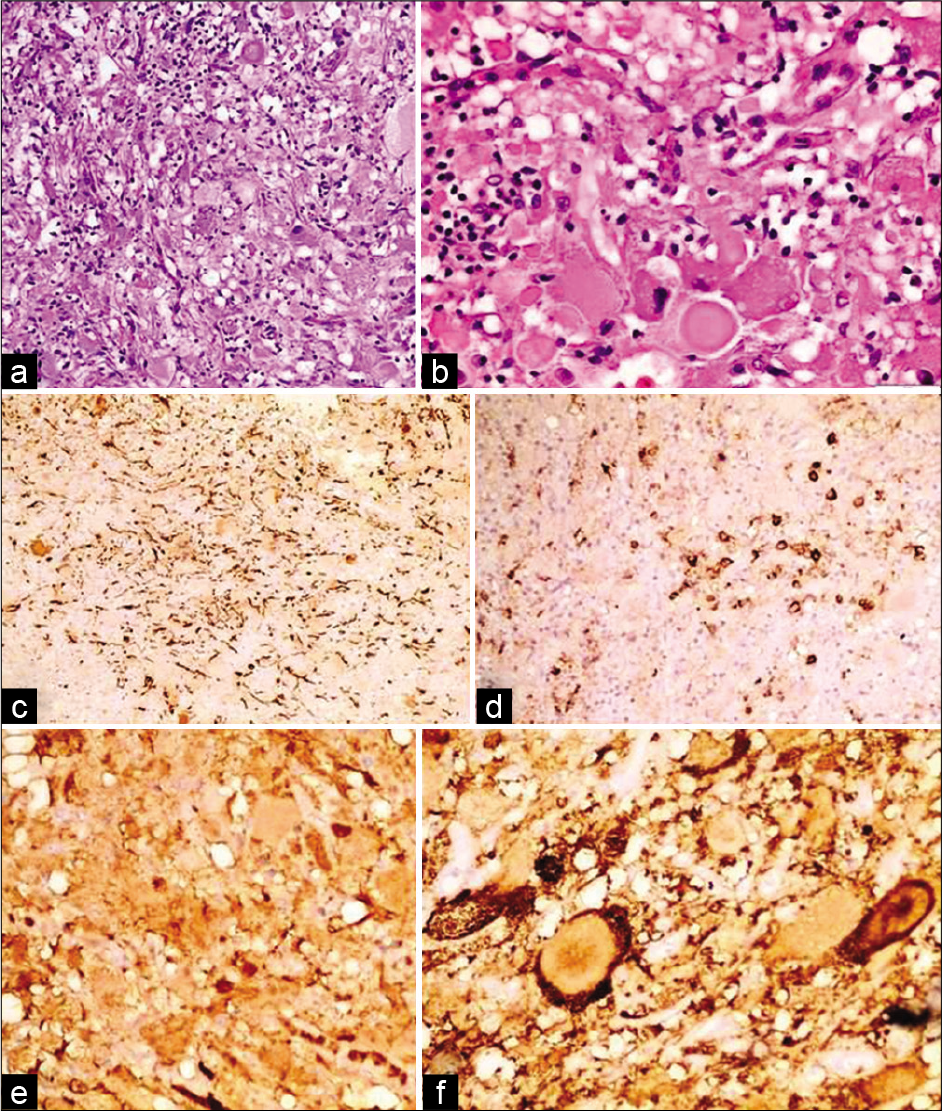
Histopathological examination revealed ganglioglioma (WHO Grade I). Microscopic examination showed clusters of abnormal neurons which exhibit enlarged hyperchromatic nuclei and some are binucleated. Between the neurons, there are dissecting fibrillary cells highlighted by GFAP. No necrosis, mitosis or endovascular proliferation were seen. The abnormal neurons are immunoreactive for S-100 and Synaptophysin, negative for EMA and CEA. CD68 highlight scattered macrophages in the background. KI67 was less than 1% [
Figure 2:
Histopathologic examination of the tumor revealed clusters of abnormal neurons which exhibit enlarged hyperchromatic nuclei and some are binucleated in H&E ×10 (a) and H&E ×40 (b). Between the neurons, there are dissecting fibrillary cells highlighted by GFAP (c). The abnormal neurons are negative for EMA (d) and positive for S-100 (e) and Synaptophysin (f).
DISCUSSION
Gangliogliomas involving the optic chiasm are extremely rare. To the best of our knowledge, 20 cases, including ours, have been reported in the literature [
On CT scan, gangliogliomas appear as hypodense lesions with calcification and a variable contrast enhancement pattern. The MRI findings are nonspecific and mimic pilocytic astrocytomas, as at this site they are indolent, slow-growing neoplasms, and their presenting symptoms vary depending on location.[
Histologically, gangliogliomas are considered benign tumors, usually classified as WHO Grade 1. The cases of primary atypical (WHO Grade 2) and anaplastic (WHO Grade 3) gangliogliomas are more rare.[
Most CNS gangliogliomas are well circumscribed. Therefore, surgical excision is possible, resulting in a generally favorable prognosis.[
CONCLUSION
Ganglioglioma of the optic chiasm is extremely rare pathologies and a presurgical diagnosis of ganglioglioma is very infrequent with confused radiologically with low-grade pilocytic astrocytomas. We report this case due to its relative rarity and to broaden the differential diagnoses of lesions arising in the optic chiasm.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albayrak R, Albayram S, Port J, Degirmenci B, Acar M, Yucel A. Ganglioglioma of the right optic tract: Case report and review of the literature. Magn Reson Imaging. 2000. 22: 1047-51
2. Allen JC, Judkins AR, Rosenblum MK, Biegel JA. Atypical teratoid/rhabdoid tumor evolving from an optic pathway ganglioglioma: Case study. Neuro Oncol. 2006. 8: 79-82
3. Chilton J, Caughron MR, Kepes JJ. Ganglioglioma of the optic chiasm: Case report and review of the literature. Neurosurgery. 1990. 26: 1042-5
4. Cogan DG, Poppen JL, Hicks SP. Ganglioneuroma of chiasm and optic nerves. Arch Ophthalmol. 1961. 65: 481-2
5. Courville CB. Ganglioglioma, tumor of the central nervous system: Review of the literature and report of two cases. Arch Neurol Psychiatr. 1930. 24: 439-91
6. Dash RC, Provenzale JM, McComb RD, Perry DA, Longee DC, McLendon RE. Malignant supratentorial ganglioglioma (ganglion cell-giant cell glioblastoma): A case report and review of the literature. Arch Pathol Lab Med. 1999. 123: 342-5
7. Gupta R, Suri V, Arora R, Sharma MC, Mishra S, Singh M. Suprasellar ganglioglioma presenting with diabetes insipidus in a young boy: A rare clinical presentation. Childs Nerv Syst. 2010. 26: 255-8
8. Haddad SF, Moore SA, Menezes AH, Vangilder JC. Ganglioglioma: 13 years of experience. Neurosurgery. 1992. 31: 171-8
9. Jalali R, Deopujari CE, Bhutani R, Suhas U, Rajasekharan P, Kane SV. Suprasellar ganglioglioma with unusual diffuse involvement of the entire optico-chiasmal hypothalamic pathway. J Cancer Res Ther. 2008. 4: 140-3
10. Lantos PL, Vandenberg SR, Kleihues P, Graham DI, Lantos PL.editors. Tumors of the nervous system. Greenfield’s Neuropathology. London: Arnold; 1997. p. 663-77
11. Liu GT, Galetta SL, Rorke LB, Bilaniuk LT, Vojta DD, Molloy PT. Gangliogliomas involving the optic chiasm. Neurology. 1996. 46: 1669-73
12. Lowes M, Bojsen-Moller M, Vorre P, Hedegaard O. An evaluation of gliomas of the anterior visual pathways: A 10-year survey. Acta Neurochir (Wien). 1978. 43: 201-6
13. Lu WY, Goldman M, Young B, Davis DG. Optic nerve ganglioglioma. Case report. J Neurosurg. 1993. 78: 979-82
14. Pant I, Suri V, Chaturvedi S, Dua R, Kanodia AK. Ganglioglioma of optic chiasma: Case report and review of literature. Childs Nerv Syst. 2006. 22: 717-20
15. Rodriguez LA, Edwards MS, Levin VA. Management of hypothalamic gliomas in children: An analysis of 33 cases. Neurosurgery. 1990. 26: 242-7
16. Shuangshoti S, Kirsch E, Bannan P, Fabian VA. Ganglioglioma of the optic chiasm: Case report and review of the literature. AJNR Am J Neuroradiol. 2000. 21: 1486-9
17. Spennato P, Giordano M, Ruggiero C, Aliberti F, Buonocore MC, Nastro A. Optic pathway ganglioglioma with intraventricular cyst. J Neurooncol. 2011. 102: 499-508
18. Sugita Y, Sawada M, Munemitsu T, Higashi T, Hojo M. Discrepancy between radiological and histological findings in ganglioglioma of the optic chasm: Case report. Surg Neurol Int. 2017. 8: 146
19. Sugiyama K, Goishi J, Sogabe T, Uozumi T, Hotta T, Kiya K. Ganglioglioma of the optic pathway: A case report. Surg Neurol. 1992. 37: 22-5
20. Vajramani GV, Dambatta S, Walker M, Grundy PL. Multiple gangliogliomas of the optic pathway. Br J Neurosurg. 2006. 20: 428-30






James Ausman
Posted November 23, 2020, 7:14 am
This is an outstanding unique case observation of a rare lesion. It is well written, informative, and nicely supported by a relevant literature search. The authors are to be congratulated for their determination to pursue an answer for this patient to a vision threatening problem. The surgical approach is thoughtful, and leads the reader to admire its required meticulous surgical technique in a highly sensitive area for its planned surgical attack. It is an example to all to pursue a logical solution with good judgment to a disease using all available sources to provide help to the patient. Excellent work and achievement. Thank you.