- Department of Orthopaedics, Byramjee Jeejeebhoy Medical College and Sassoon General Hospital, Pune, Maharashtra, India.
Correspondence Address:
Divya Tomer, Department of Orthopaedics, Byramjee Jeejeebhoy Medical College and Sassoon General Hospital, Pune, Maharashtra, India.
DOI:10.25259/SNI_207_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Girish Bartakke, Mohit Muttha, Divya Tomer, Shubham Ashok Kadam. Giant cell tumor of sacral vertebra in an adolescent without neurodeficit: A case report and review of the literature. 31-Mar-2023;14:121
How to cite this URL: Girish Bartakke, Mohit Muttha, Divya Tomer, Shubham Ashok Kadam. Giant cell tumor of sacral vertebra in an adolescent without neurodeficit: A case report and review of the literature. 31-Mar-2023;14:121. Available from: https://surgicalneurologyint.com/surgicalint-articles/12221/
Abstract
Background: Giant cell tumors (GCTs) are locally aggressive benign primary bone tumors that rarely occur in the spine. Their treatment methods include denosumab, bisphosphonates, and/or different surgical techniques. Here, we present the successful treatment of a sacral GCT in a 13 years old.
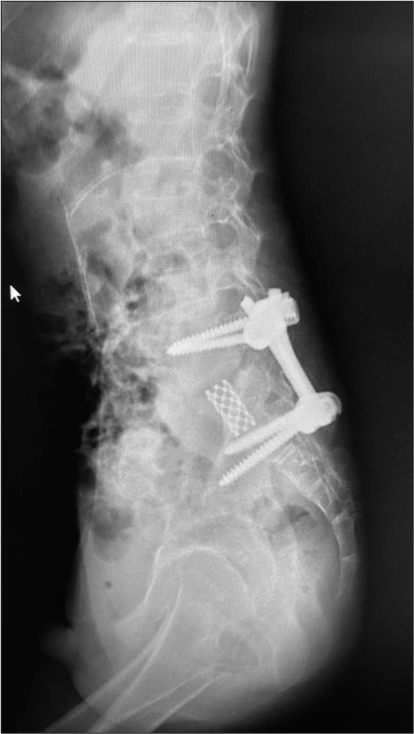
Case Description: A 13-year-old male presented with back pain and paraparesis of 3-week duration. Radiological studies demonstrated an S1 lytic lesion. He underwent an excisional biopsy and anterior and posterior resection combined with a lumbopelvic fusion. One year later, there has been no tumor recurrence.
Conclusion: We successfully treated an S1 sacral GCT in a 13-year-old male utilizing a wide anteriorand posterior excision combined with a lumbopelvic fusion.
Keywords: Denosumab, Giant cell tumors, Primary bone tumors, Primary spinal tumors, Sacral tumors
INTRODUCTION
Giant cell tumors (GCTs) are benign typically long bone lesions that rarely involve the spine. They often occur in patients between the ages of 20–45 years and just 1.7–8.2% involve the sacrum.[
CASE REPORT
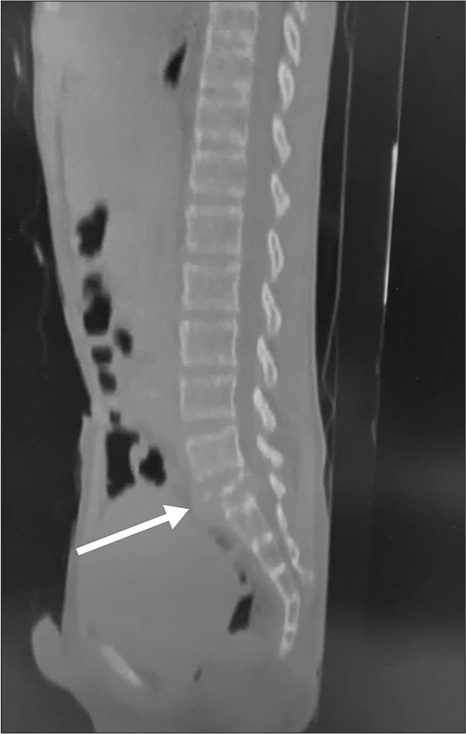
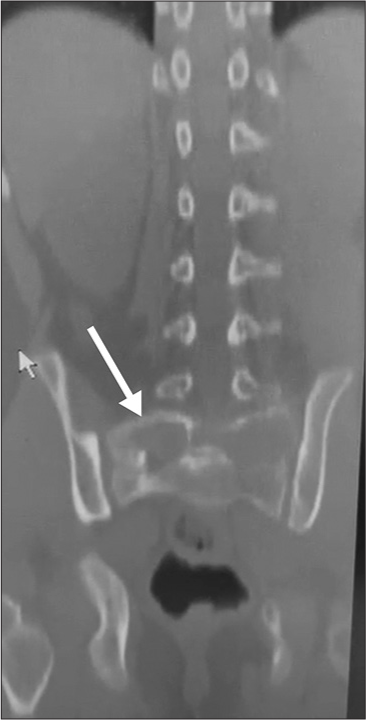
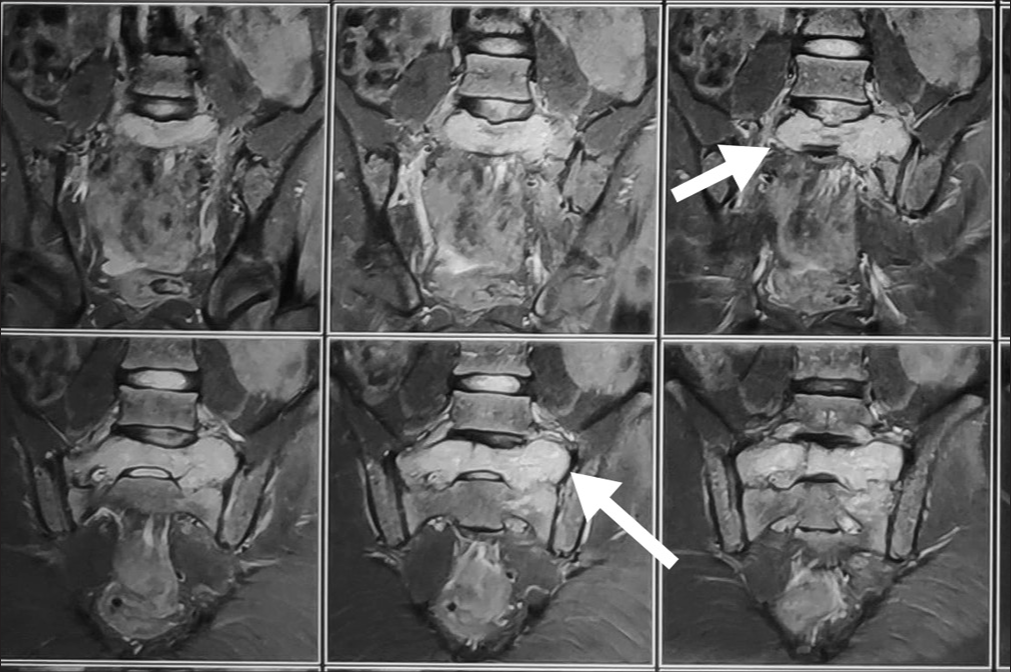
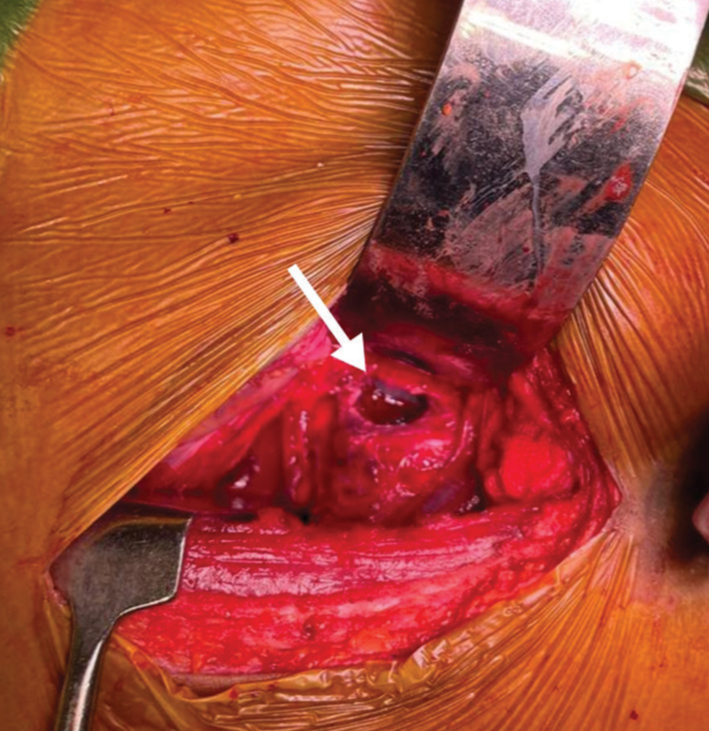
A 13-year-old male presented with 3 weeks of progressive low back pain and paraparesis. The X-rays and computed tomography (CT) scan of the lumbosacral spine showed a lytic S1 sacral alar lesion [
DISCUSSION
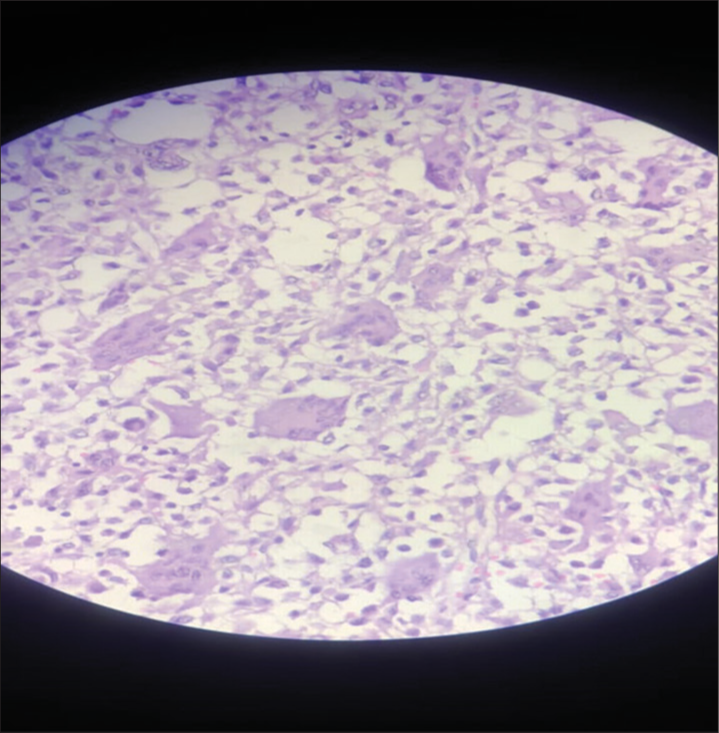
GCTs are typically lytic vertebral lesions. The main differential diagnoses include hyperparathyroidism, aneurysmal bone cyst, osteoblastoma, and tuberculosis. Pathologically, they are identified based on their multinucleated osteoclasts in a spindle cell matrix (i.e. should be confirmed by biopsy and/or surgical resection).[
Treatment after GCT surgery with adjunctive denosumab
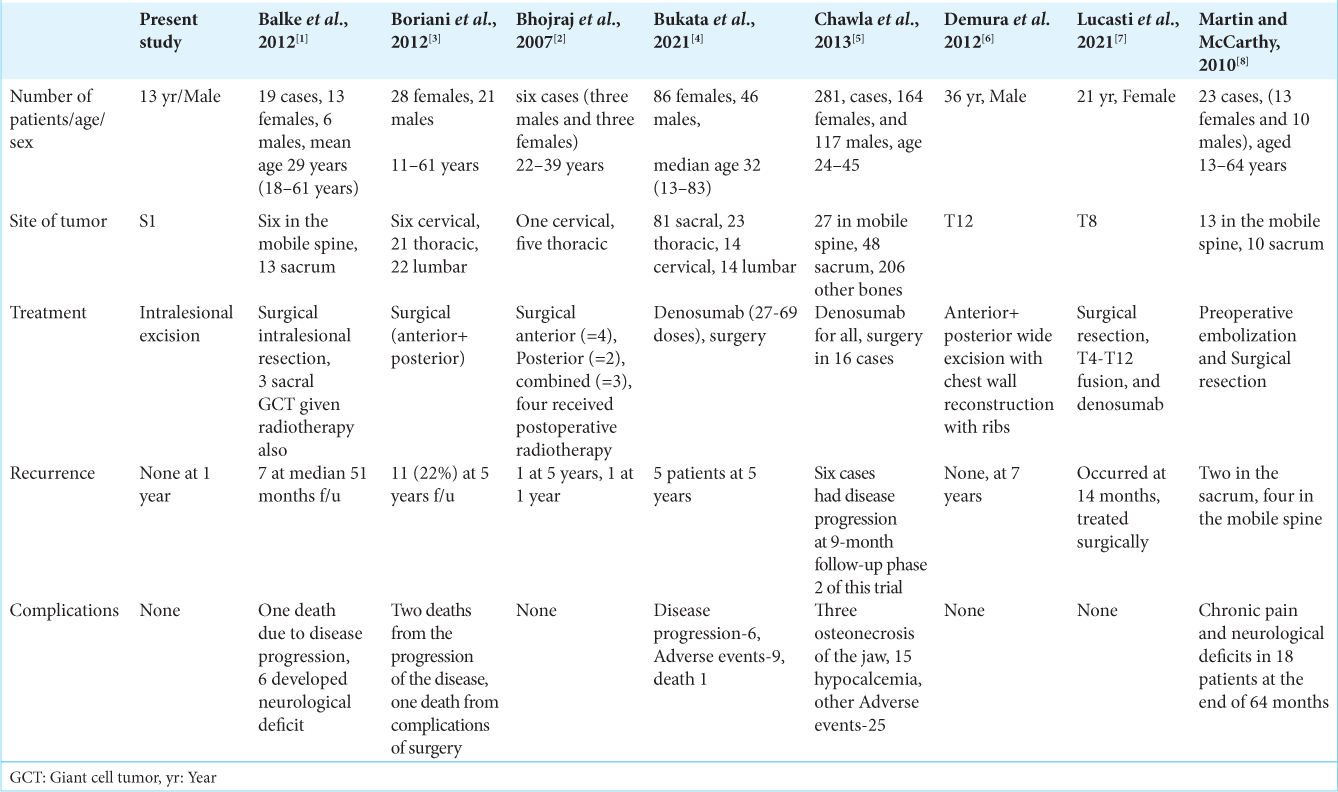
Several studies demonstrated the efficacy of denosumab utilized following GCT spinal surgery[
Radiation therapy (RT) following surgery for GCT
Radiotherapy following intralesional GCT resection helps to prevent tumor recurrence. Bhojraj et al. Observed no instances of sarcoma or other side effects when using radiation to treat GCT (i.e., <50 Gy).[
Local recurrence rates of spinal/sacral GCT
Boriani et al.[
CONCLUSION
A 13-year-old male diagnosed with a GCT S1 lytic lesion successfully underwent circumferential tumor resection with lumbopelvic fixation.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Balke M, Henrichs MP, Gosheger G, Ahrens H, Streitbuerger A, Koehler M. Giant cell tumors of the axial skeleton. Sarcoma. 2012. 2012: 410973
2. Bhojraj SY, Nene A, Mohite S, Varma R. Giant cell tumor of the spine: A review of 9 surgical interventions in 6 cases. Indian J Orthop. 2007. 41: 146-50
3. Boriani S, Bandiera S, Casadei R, Boriani L, Donthineni R, Gasbarrini A. Giant cell tumor of the mobile spine: A review of 49 cases. Spine (Phila Pa 1976). 2012. 37: E37-45
4. Bukata SV, Blay JY, Rutkowski P, Skubitz K, Henshaw R, Seeger L. Denosumab treatment for giant cell tumor of the spine including the sacrum. Spine (Phila Pa 1976). 2021. 46: 277-84
5. Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, Phase 2 study. Lancet Oncol. 2013. 14: 901-8
6. Demura S, Kawahara N, Murakami H, Akamaru T, Kato S, Oda M. Giant cell tumor expanded into the thoracic cavity with spinal involvement. Orthopedics. 2012. 35: e453-6
7. Lucasti C, Patel D, Hawayek B, Maraschiello M, Kowalski J. Giant cell tumor of the thoracic spine causing acute paraplegia-a case report. J Spine Surg. 2021. 7: 208-13
8. Martin C, McCarthy EF. Giant cell tumor of the sacrum and spine: Series of 23 cases and a review of the literature. Iowa Orthop J. 2010. 30: 69-75
9. Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am. 2006. 37: 35-51
10. Werner M. Giant cell tumour of bone: Morphological, biological and histogenetical aspects. Int Orthop. 2006. 30: 484-9