- Department of Neurosurgery, University of Athens Medical School, “Evangelismos” General Hospital, National and Kapodistrian University of Athens, Athens, Greece
- Department of Imaging, National and Kapodistrian University of Athens, Athens, Greece
- Department of Pathology, Children’s Hospital “Aghia Sofia”, Athens, Greece
- Department of First Department of Pediatrics, National and Kapodistrian University of Athens, Athens, Greece
- Department of Neurosurgery, Children’s Hospital “Aghia Sophia”, Athens, Greece
Correspondence Address:
Dimitrios Giakoumettis
Department of Neurosurgery, Children’s Hospital “Aghia Sophia”, Athens, Greece
DOI:10.25259/SNI-85-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Dimitrios Giakoumettis, Ioannis Nikas, Kalliopi Stefanaki, Antonis Kattamis, George Sfakianos, Marios S. Themistocleous. Giant intracranial congenital hemangiopericytoma/solitary fibrous tumor: A case report and literature review. 24-Apr-2019;10:75
How to cite this URL: Dimitrios Giakoumettis, Ioannis Nikas, Kalliopi Stefanaki, Antonis Kattamis, George Sfakianos, Marios S. Themistocleous. Giant intracranial congenital hemangiopericytoma/solitary fibrous tumor: A case report and literature review. 24-Apr-2019;10:75. Available from: http://surgicalneurologyint.com/surgicalint-articles/9278/
Abstract
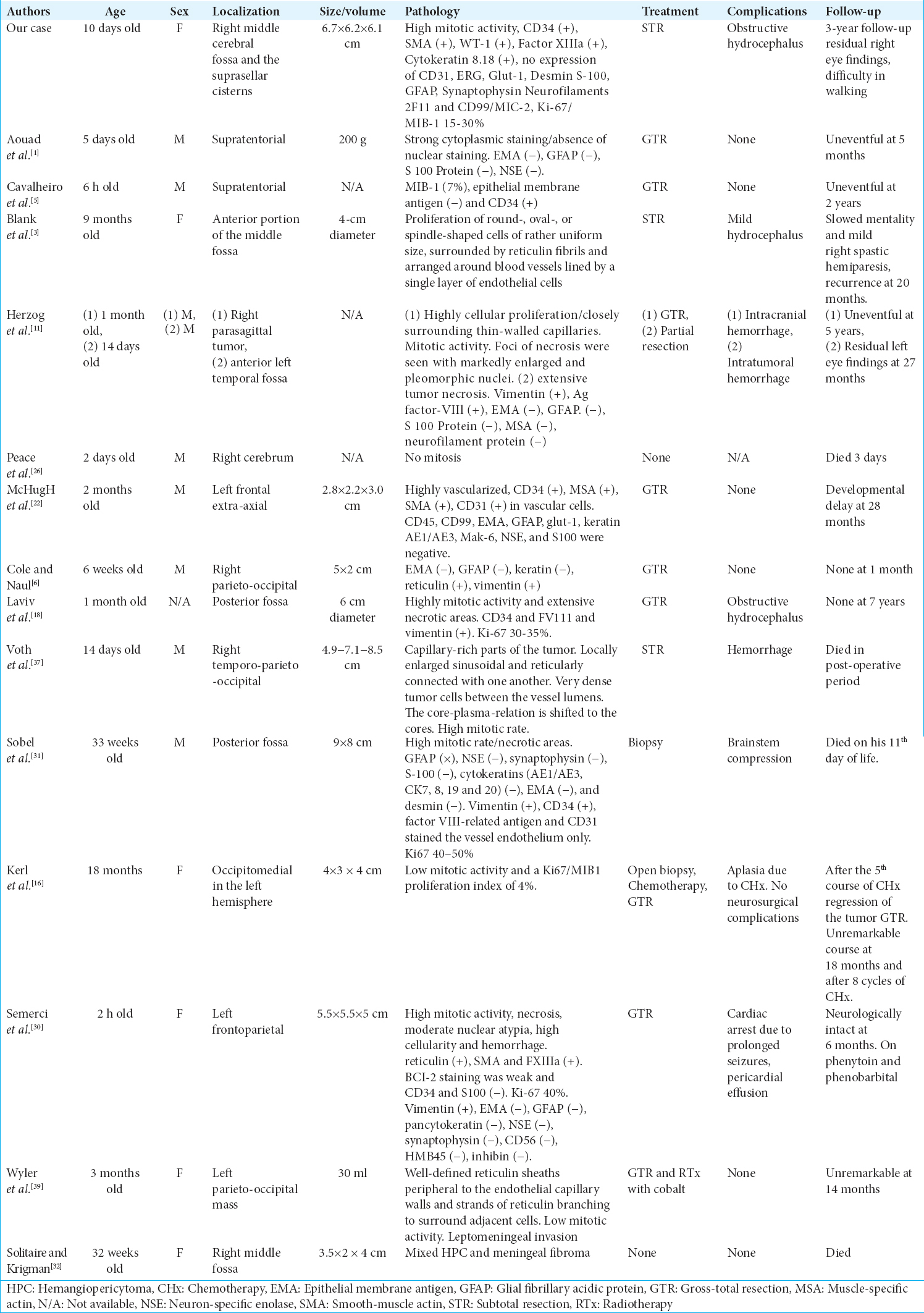
Background:Hemangiopericytoma and solitary fibrous tumor (HPC/SFT) are considered to be one category according to the WHO 2016 classification of central nervous system tumors. HPC/SFT are subdivided into infantile (congenital) and adult type. Both are extremely rare entities, with little knowledge about etiology, prognosis, and optimal therapeutic strategy.
Case Description:A 10-day-old girl was referred to our neurosurgical department due to hypotonia, palsy of the right oculomotor nerve, and prominent frontal fontanel. Imaging studies revealed a large occupying mass in the right middle cerebral fossa and the suprasellar cisterns. Only a subtotal resection of the tumor was possible, and postoperatively, she underwent chemotherapy (CHx). After a 3-year follow-up, the girl has minimum neurologic signs and receives no medications, and she can walk when she is supported.
Conclusion:Congenital HPC/SFT is considered to have a benign behavior with a good prognosis. Treatment with gross total resection, when it is feasible, is the key to a good prognosis and low rates of recurrence. However, there is no consensus on the therapeutic strategy of a HPC/SFT, which is difficult to be completely resected. Literature lacks a therapeutic algorithm for these tumors, and thus, more clinical studies are needed to reach a consensus.
Keywords: Congenital, hemangiopericytoma, intracranial, solitary fibrous tumor
INTRODUCTION
It is known that congenital brain tumors are very rare with an incidence of 1.1–3.6/100,000 newborns.[
CASE REPORT
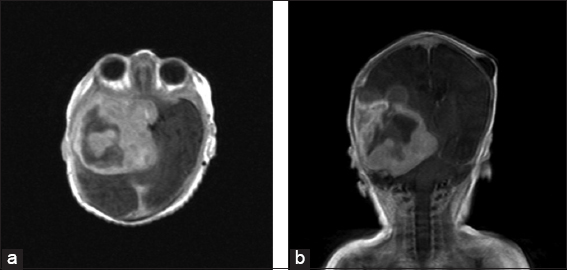
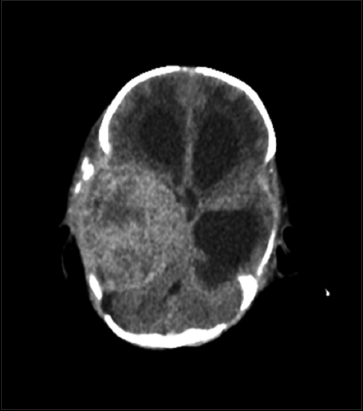
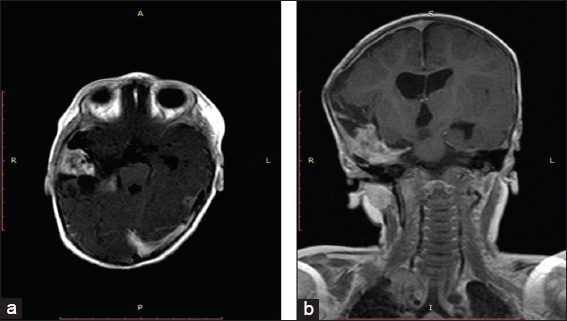
A 10-day-old girl was referred to our neurosurgical department from the neonatal intensive care unit where it was being treated since her 3rd day after birth due to jaundice. She presented with hypotonia, palsy of the right oculomotor nerve, and prominent frontal fontanel; a cerebral ultrasound and subsequently a computed tomography (CT) scan were performed and revealed a large hyperdense space-occupying mass in the right middle cerebral fossa and the suprasellar cisterns. Magnetic resonance imaging (MRI) demonstrated a tumor with marked inhomogeneous enhancement, with mixed cystic and solid components with dimensions of 6.7 cm × 6.2 cm × 6.1 cm [
DISCUSSION
The incidence of congenital tumors is 0.34 per one million births, and infantile HPCs are extremely rare with an incidence of <1% of all CNS tumors.[
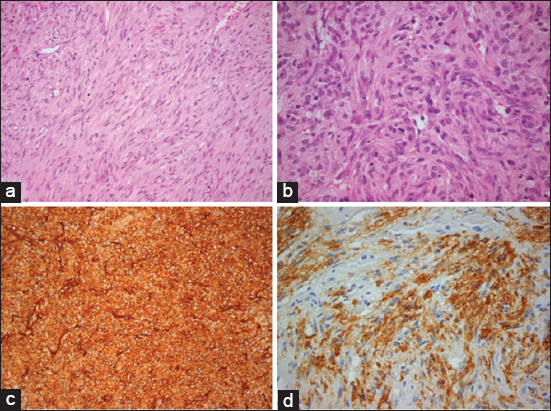
In our case, the neoplasm shows morphological heterogeneity and is composed of cellular areas of the short bundles of spindle, stellate, and ovoid cells with eosinophilic cytoplasm and a round nucleus with fine chromatin without nucleolus and with mild-to-moderate nuclear atypia. A moderate-to-brisk mitotic activity of 12–40 mitoses/10 high-power fields (hpf) × 40 and 2–6 mitoses/hpf × 40 was recognized [
Figure 4
(a) Cellular areas of short bundles of spindle cells with an eosinophilic cytoplasm and a round nucleus with fine chromatin without nucleolus and with mild-to-moderate nuclear atypia. (b) Heterogeneous cellular density with cellular heterogeneity and obvious mitoses in a cellular area of the neoplasm. (c) Immunohistochemical expression of CD34 in the neoplastic cells mainly membranous and cytoplasmic. (d) A heterogeneous expression of smooth muscle actin in bundles of spindle cells.
In
CONCLUSIONS
Infantile HPC/SFT is considered to have a benign behavior with a good prognosis. Treatment with GTR is the key to a good prognosis and low rates of recurrence. Nevertheless, GTR is not always feasible. There is no consensus on the therapeutic strategy of a HPC/SFT, which is difficult to be completely resected. CHx before surgery has been proven useful, to make the tumor operable. Moreover, it has been successfully applied as an adjuvant therapy after surgery, or in case of recurrence. RTx has also been used in treating these tumors, but there have been studies that support its ineffectiveness. Literature lacks a therapeutic algorithm for these tumors, and thus, more clinical studies are needed to reach a consensus. In our case, a subtotal resection was performed, which was postoperatively complicated with obstructive hydrocephalus. After a ventriculoperitoneal shunt operation, the patient received CHx.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aouad N, Vital C, Rivel J, Ramsoubramanian K, Santosh S, Chowdry O. Giant supratentorial meningeal haemangiopericytoma in a newborn. Acta Neurochir (Wien). 1991. 112: 154-6
2. Bien E, Stachowicz-Stencel T, Godzinski J, Balcerska A, Izycka-Swieszewska E, Kazanowska B. Retrospective multi-institutional study on hemangiopericytoma in polish children. Pediatr Int. 2009. 51: 19-24
3. Blank W, Spring A, Giesen H, Artmann H. Intracranial hemangiopericytoma in a child. Klin Padiatr. 1988. 200: 422-5
4. Bouvier C, Métellus P, de Paula AM, Vasiljevic A, Jouvet A, Guyotat J. Solitary fibrous tumors and hemangiopericytomas of the meninges:Overlapping pathological features and common prognostic factors suggest the same spectrum of tumors. Brain Pathol. 2012. 22: 511-21
5. Cavalheiro S, Sparapani FV, Moron AF, da Silva MC, Stávale JN. Fetal meningeal hemangiopericytoma. Case report. J Neurosurg. 2002. 97: 1217-20
6. Cole JC, Naul LG. Intracranial infantile hemangiopericytoma. Pediatr Radiol. 2000. 30: 271-3
7. Coppa ND, Raper DM, Zhang Y, Collins BT, Harter KW, Gagnon GJ. Treatment of malignant tumors of the skull base with multi-session radiosurgery. J Hematol Oncol. 2009. 2: 16-
8. del Rosario ML, Saleh A. Preoperative chemotherapy for congenital hemangiopericytoma and a review of the literature. J Pediatr Hematol Oncol. 1997. 19: 247-50
9. Fernandez-Pineda I, Parida L, Jenkins JJ, Davidoff AM, Rao BN, Rodriguez-Galindo C. Childhood hemangiopericytoma:Review of st jude children's research hospital. J Pediatr Hematol Oncol. 2011. 33: 356-9
10. Ferrari A, Casanova M, Bisogno G, Mattke A, Meazza C, Gronchi A. Hemangiopericytoma in pediatric ages:A report from the italian and german soft tissue sarcoma cooperative group. Cancer. 2001. 92: 2692-8
11. Herzog CE, Leeds NE, Bruner JM, Baumgartner JE. Intracranial hemangiopericytomas in children. Pediatr Neurosurg. 1995. 22: 274-9
12. Hodaie M, Becker L, Teshima I, Rutka JT. Total resection of an intracerebral hemangioendothelioma in an infant. Case report and review of the literature. Pediatr Neurosurg. 2001. 34: 104-12
13. Jänisch W, Haas JF, Schreiber D, Gerlach H. Primary central nervous system tumors in stillborns and infants. Epidemiological considerations. J Neurooncol. 1984. 2: 113-6
14. Jellinger K, Sunder-Plassmann M. Connatal intracranial tumours. Neuropadiatrie. 1973. 4: 46-63
15. Jha N, McNeese M, Barkley HT, Kong J. Does radiotherapy have a role in hemangiopericytoma management?Report of 14 new cases and a review of the literature. Int J Radiat Oncol Biol Phys. 1987. 13: 1399-402
16. Kerl K, Sträter R, Hasselblatt M, Brentrup A, Frühwald MC. Role of neoadjuvant chemotherapy in congenital intracranial haemangiopericytoma. Pediatr Blood Cancer. 2011. 56: 161-3
17. Kirk IR, Dominguez R, Castillo M. Congenital primary cerebral angiosarcoma:CT, US, and MR findings. Pediatr Radiol. 1992. 22: 134-5
18. Laviv Y, Michowitz S, Schwartz M. Neonatal intracranial hemangiopericytoma:A 7-year follow-up. Acta Neurochir (Wien). 2012. 154: 637-8
19. Lee DY, Kim YM, Yoo SJ, Cho BK, Chi JG, Kim IO. Congenital glioblastoma diagnosed by fetal sonography. Childs Nerv Syst. 1999. 15: 197-201
20. Leins AM, Kainer F, Weis S. Sonography and neuropathology of a congenital brain tumor:Report of a rare incident. Ultrasound Obstet Gynecol. 2001. 17: 245-7
21. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. The 2016 world health organization classification of tumors of the central nervous system:A summary. Acta Neuropathol. 2016. 131: 803-20
22. McHugh BJ, Baranoski JF, Malhotra A, Vortmeyer AO, Sze G, Duncan CC. Intracranial infantile hemangiopericytoma. J Neurosurg Pediatr. 2014. 14: 149-54
23. Mena H, Ribas JL, Enzinger FM, Parisi JE. Primary angiosarcoma of the central nervous system. Study of eight cases and review of the literature. J Neurosurg. 1991. 75: 73-6
24. Nakayama K, Nakamura Y. Localization of congenital glioblastomas in the Japanese:A case report and review of the literature. Childs Nerv Syst. 2002. 18: 149-52
25. Pandey M, Kothari KC, Patel DD. Haemangiopericytoma:Current status, diagnosis and management. Eur J Surg Oncol. 1997. 23: 282-5
26. Peace RJ. A congenital neoplasm of the brain of a newborn infant;report of a case with necropsy. Am J Clin Pathol. 1954. 24: 1272-5
27. Rodriguez-Galindo C, Ramsey K, Jenkins JJ, Poquette CA, Kaste SC, Merchant TE. Hemangiopericytoma in children and infants. Cancer. 2000. 88: 198-204
28. Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010. 113: 333-9
29. Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma:Long-term outcome revisited. Clinical article. J Neurosurg. 2011. 114: 747-55
30. Semerci SY, Demirel G, Vatansever B, Gundogdu S, Bolukbasi F, Oran G. Urgent surgical management of congenital intracranial hemangiopericytoma in a preterm neonate. Br J Neurosurg. 2017. 22: 1-3
31. Sobel G, Halász J, Bogdányi K, Szabó I, Borka K, Molnár P. Prenatal diagnosis of a giant congenital primary cerebral hemangiopericytoma. Pathol Oncol Res. 2006. 12: 46-9
32. Solitare GB, Krigman MR. Congenital intracranial neoplasm. a case report and review of the literature. J Neuropathol Exp Neurol. 1964. 23: 280-92
33. Staples JJ, Robinson RA, Wen BC, Hussey DH. Hemangiopericytoma the role of radiotherapy. Int J Radiat Oncol Biol Phys. 1990. 19: 445-51
34. Sultan I, Casanova M, Al-Jumaily U, Meazza C, Rodriguez-Galindo C, Ferrari A. Soft tissue sarcomas in the first year of life. Eur J Cancer. 2010. 46: 2449-56
35. Suzuki Y, Yoshida YK, Shirane R, Yoshimoto T, Watanabe M, Moriya T. Congenital primary cerebral angiosarcoma. Case report. J Neurosurg. 2000. 92: 466-8
36. Toren A, Perlman M, Polak-Charcon S, Avigad I, Katz M, Kuint Y. Congenital hemangiopericytoma/infantile myofibromatosis:Radical surgery versus a conservative “wait and see” approach. Pediatr Hematol Oncol. 1997. 14: 387-93
37. Voth D, Schröder JM, Gutjahr P, Stopfkuchen H, Kühnert A, Günther R. Intracranial hemangiopericytoma in a newborn (author's transl). Z Kinderchir. 1981. 32: 85-90
38. Winters JL, Wilson D, Davis DG. Congenital glioblastoma multiforme:A report of three cases and a review of the literature. J Neurol Sci. 2001. 188: 13-9
39. Wyler AR, Hered J, Smith JR, Loeser JD. Subarachnoid hemorrhage in infancy due to brain tumor. Arch Neurol. 1973. 29: 447-8