- Division of Neurosurgery, Santa Paula Hospital, São Paulo, SP, Brazil
Correspondence Address:
Daniel Andrade Gripp
Division of Neurosurgery, Santa Paula Hospital, São Paulo, SP, Brazil
DOI:10.4103/2152-7806.179573
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gripp DA, Fábio Jundy Nakasone, Marcos Vinícius Calfat Maldaun, de Aguiar PH P, Mathias LR. Giant pseudoaneurysm originated from distal middle cerebral artery dissection treated by trapping under sensitive evoked potential and motor evoked potential monitoring: Case report and discussion. Surg Neurol Int 01-Apr-2016;7:
How to cite this URL: Gripp DA, Fábio Jundy Nakasone, Marcos Vinícius Calfat Maldaun, de Aguiar PH P, Mathias LR. Giant pseudoaneurysm originated from distal middle cerebral artery dissection treated by trapping under sensitive evoked potential and motor evoked potential monitoring: Case report and discussion. Surg Neurol Int 01-Apr-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/giant-pseudoaneurysm-originated-from-distal-middle-cerebral-artery-dissection-treated-by-trapping-under-sensitive-evoked-potential-and-motor-evoked-potential-monitoring-case-report-and-discussion/
Abstract
Background:Dissecting giant pseudoaneurysm of the middle cerebral artery (MCA) is a rare lesion often presenting challenges to neurosurgical teams dealing with this specific pathology. Giant pseudoaneurysm originating from a dissecting distal segment of the MCA treated with aneurysm trapping under motor and sensitive evoked potential monitoring with a successful outcome is presented in the article followed by a brief discussion on the subject.
Case Description:A case of a previously healthy young female patient admitted at the emergency room of Santa Paula Hospital with a history of a sudden headache and syncope, dysphasia, and Grade 4 right hemiparesis due to a large brain hemorrhage secondary to a 25 mm ruptured pseudoaneurysm originated from a distal left MCA dissecting segment is described. Because the patient risked neurological worsening, aneurysm was treated with parent and efferent vessel trapping technique and no changes on the sensitive and motor evoked potential (MEP) from baseline informed on this decision. Hemorrhage was completely drained after aneurysm was secured.
Conclusion:Neurophysiological sensitive and MEP monitoring, on this specific case was a valuable tool and informed on the decision of trapping of this large vascular lesion.
Keywords: Middle cerebral artery, neurophysiological monitoring, pseudoaneurysm, trapping
INTRODUCTION
Patients with complex middle cerebral artery (MCA) aneurysms – dissecting or giant lesions – may present with intracranial hemorrhage, mass effects, epilepsy or cerebral ischemia; in addition, aneurysm may be incidentally discovered. Often with an unfavorable anatomical morphology of the neck and dome, complex MCA aneurysms present a unique challenge to medical teams.
We describe here a giant pseudoaneurysm case originating from a dissection of the left parietal-occipital branch of the MCA atypically presenting intracranial hemorrhage, surgically treated by trapping of parent and efferent vessels under neurophysiological monitoring. We will present here a brief revision and discussion referencing the literature on dissecting and giant aneurysms and strategies for their treatment.
CASE REPORT
A previously healthy 29-year-old female patient was admitted to the emergency room of Santa Paula Hospital, Sao Paulo, reporting a sudden headache and syncope associated with mild dysphasia and weakness of right arm and leg. Upon admission, she was graded a Glasgow Coma Scale of 13 and a Grade 4 right hemiparesis. According to her husband, she had not suffered any trauma. The patient was hemodynamically stable and reported on regular use of oral contraceptive.
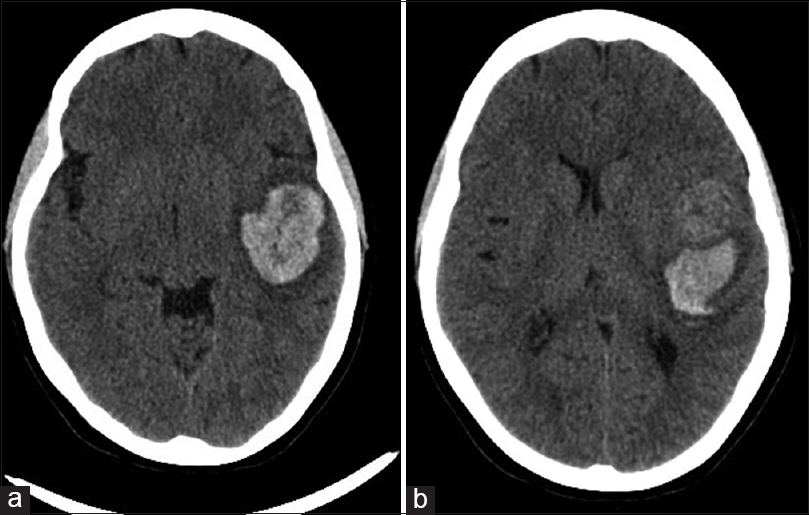
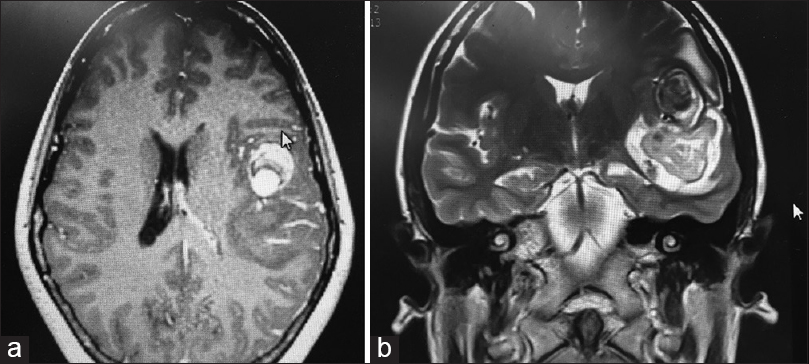
When the patient was submitted to a computed tomography (CT) scan, the following were detected: A nodular hyperdense frontotemporal image anterior to a large left side predominantly temporal hematoma associated to edema and signs of subfalcine and transtentorial herniation, and a midline shift of approximately 6 mm [
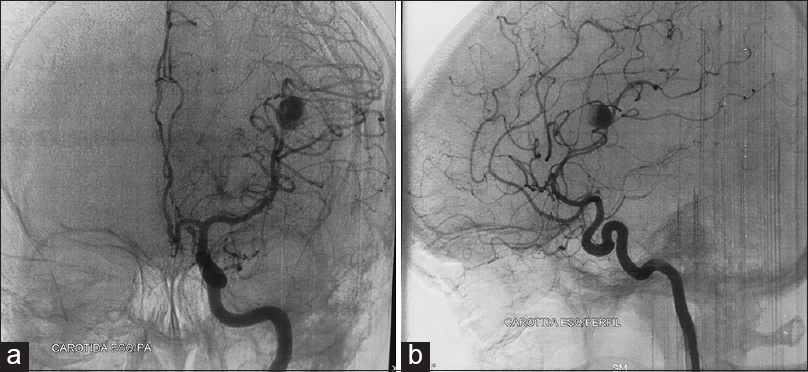
Surgery under sensitive evoked potential (SEP) and motor evoked potential (MEP) monitoring was performed on an urgent basis given the risk of further neurological worsening.
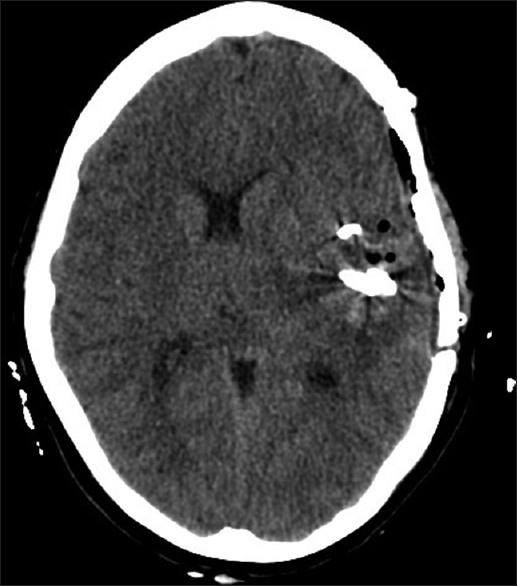
A left side large frontotemporal craniotomy was performed along with wide Sylvian fissure opening and complete aneurysm dome and neck dissection. Aneurysm was trapped, and no reduction on neurophysiological parameters from baseline could be noted afterward, thus prompting us not to do a bypass. After the opening of the lesion wall, no bleeding was noted and aneurysmectomy was conducted given the large dome had considerable mass effect on the adjacent brain. Draining of the temporal hemorrhage considerably alleviated regional cerebral edema. Immediate postoperative CT scan showed complete drainage of the temporal bleeding and less midline shift than the previous scan [
No vasospasm was detected by subsequent transcranial Doppler monitoring done from postoperative day 1. Intense rehabilitation led to complete amelioration of hemiparesis and near total dysphasia improvement, upon discharge from the hospital.
DISCUSSION
Complex aneurysms are defined as having large (10–24 mm in diameter) or giant (diameter ≥25 mm) size or nonsaccular morphology (fusiform, dissecting, or serpentine);[
The literature on dissecting intracranial aneurysms frequently describes them as rare lesions. They are most often related to traumatic events, spontaneous lesions without a clearly identifiable cause are uncommon. They are associated to intracranial vessel dissection, either in the carotid or basilar circulation or on its branches.
When affecting the posterior cerebral circulation, dissecting aneurysms present more often as subarachnoid hemorrhage whereas, in the carotid circulation, the occurrence is more common in the form of cerebral ischemia due to stenosis or vessel occlusion due to thrombus formation. Occurrence in the posterior vessels has been more frequently described and documented. With an increasing number of case reports of cerebral hemorrhage due to these types of lesions in the anterior circulation being more frequently published, we suspect their prevalence may be more significant than we previously thought. A large thrombosed aneurysm associated to MCA distal segment dissection is probably a rare event. In 2002, a Japanese cooperative group reported on a total of 49 dissecting aneurysm in the anterior circulation, 24% of them located on the MCA segment.[
Considering etiology, dissecting aneurysms may be related to genetic factors, primary and secondary cerebral vasculitis, fibromuscular dysplasia, infection (mycotic aneurysms), traumatic events, atherosclerosis, and hypertension. A report has linked it to migraine syndrome and repeated vascular wall edema with subsequent damage.[
Regarding giant aneurysms, occurrence is thought to relate to the same factors that originate smaller saccular lesions. The MCA is the most frequent location for giant aneurysms of the anterior circulation.[
Giant aneurysms may produce symptoms and signs that resemble those of a mass lesion, and multiple neurological deficits, seizures, and endocrine disturbances can occur depending on the location.[
Both dissecting and giant aneurysms can be accurately diagnosed using modern neuroimaging techniques. Bleeding and ischemic symptoms can be identified on CT scan and MRI imaging. In the past, when skull radiographs were more frequently used, calcification and bone erosion could be evidenced on patients harboring giant aneurysms.[
Various possibilities exist for the treatment of such lesions, either dissecting aneurysms or giant ones, and none has been well proved by the literature as the gold standard. de Divitiis et al. mentions that if there is a high risk of rebleeding (growing dissecting aneurysm, giant dissecting aneurysm or dissection associated with uncontrolled hypertension), direct treatment should be indicated.[
In general, lesions presenting with bleeding should be treated early to prevent rebleeding on patients on a favorable neurological condition. An estimated 30% chance of rebleeding of posterior circulation dissecting aneurysms has led some authors to consider a more urgent intervention when considering lesions on anterior circulation.[
Whenever possible, treatment strategies should be decided by a cerebrovascular team comprised both by neurovascular surgeons and endovascular specialists. Definite aneurysm treatment generally consists of one of the following techniques depending on each case: Endovascular therapy, parent vessel occlusion, neck reconstruction and clipping, wrapping with wall reinforcement, and trapping with or without bypass procedures. We believe direct aneurysm neck clipping and exclusion with aneurysmectomy in cases of giant lesion with mass effect to be desirable, although not always possible, especially in the case of fusiform dilatation and branches originating directly from the aneurysm wall. During surgery, superficial temporal artery branches should be preserved if a bypass is anticipated and needed due to parent and efferent vessel occlusion or ischemic symptoms.
For saccular aneurysms with a broad neck or giant size, we often use low-flow electrocoagulation to shrink the aneurysm wall or to mold the neck after temporary parent artery clipping. This is a minor procedure, but it may transform an unclippable aneurysm into a clippable one. For giant aneurysms with severe intraluminal thrombus, thrombectomy is usually required to facilitate direct clipping.[
Brain relaxation can be achieved by administration of mannitol (1 g/kg) and dexamethasone (5 mg) or cerebrospinal fluid drainage through dissection into a subarachnoid cistern. When necessary, parent artery temporary occlusion, mild hypothermia, barbiturate, or propofol have been used to increase tolerance to ischemia.
During our surgery, aneurysm was found to be an unclippable one because of its dissecting morphology. The proximal and distal M3 segments were temporary occluded. Intraoperative MEP and SEP showed no change after 30 min of occlusion during the aneurysm dissection. Therefore, the aneurysm was trapped, and an aneurysmectomy was performed. The patient recovered well without additional neurological deficits despite any revascularization procedure. Data on the use of MEP during aneurysm clipping indicate that preserved MEPs at the end of the procedure usually correspond to normal neurological postoperative examination. Mild motor deficit, in the case of preserved MEP, is usually transient and improves within a week postsurgery. Transient MEP loss is followed by a mild to transient weakness, and permanent loss of MEP is always followed by severe motor impairment, usually with early ischemic signs on immediate postoperative scans.[
The adequacy of the treatment and patency of the parent vessels after clipping may be analyzed intraoperatively using indocyanine green fluorescence angiography and/or Doppler flow measurements. Intraoperative SEP monitoring and MEP monitoring is routinely performed to detect potential injury to neurological functions. Changes in SEP or MEP have been managed by increasing the blood pressure with pressor agents or even clip repositioning according to the surgeon judgment.
Wrapping procedures consist of aneurysm wall reinforcement using muscle, cotton, Silastic sheet, Teflon, and adhesives. Figueiredo et al. have reported nine cases of clip wrapping technique on complex aneurysms with no rebleeding events on an average of 2 years follow-up,[
It is beyond our present objective to discuss endovascular therapy (coiling, stenting, diversion flow devices) of dissecting and giant aneurysms. Although there are still major limitations associated with endovascular therapy of MCA aneurysms and of giant aneurysms, in particular, a multidisciplinary discussion should always be encouraged when assessing such complex lesions.
CONCLUSION
A case of an atypical giant aneurismal lesion originating from a dissection of a distal branch (M3) of MCA was reported. Because presentation was associated with a large temporal lobe hematoma and the patient risked neurological deterioration, early surgical drainage, and trapping of aneurism was successfully conducted, and progressive improvement could be verified. Preoperative analysis of the morphology of aneurysm, vascular anatomy, hemodynamic characteristics, and intraoperative MEP and SEP monitoring was a decisive factor in choosing appropriate surgical strategies. No intraoperative MEP and SEP changes informed the decision to occlude parent and efferent vessels. The patient experienced a favorable outcome (Glasgow Outcome Scale 5), and no ischemic lesions were evident on imaging controls.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chuang MJ, Lu CH, Cheng MH. Management of middle cerebral artery dissecting aneurysm. Asian J Surg. 2012. 35: 42-8
2. Choi IS, David C. Giant intracranial aneurysms: Development, clinical presentation and treatment. Eur J Radiol. 2003. 46: 178-94
3. de Divitiis O, Di Somma A, Somma T, Cavallo LM, Marseglia M, Briganti F. Surgical clipping of a dissecting aneurysm of the precommunicating segment of the anterior cerebral artery: A case report and review of the literature. J Med Case Rep. 2015. 9: 117-
4. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: Review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997. 87: 141-62
5. Figueiredo EG, Foroni L, Monaco BA, Gomes MQ, Sterman Neto H, Teixeira MJ. The clip-wrap technique in the treatment of intracranial unclippable aneurysms. Arq Neuropsiquiatr. 2010. 68: 115-8
6. Fisher CM. Clinical syndromes in cerebral thrombosis, hypertensive hemorrhage, and ruptured saccular aneurysm. Clin Neurosurg. 1975. 22: 117-47
7. Gomes FB, Dujovny M, Umansky F, Berman SK, Diaz FG, Ausman JI. Microanatomy of the anterior cerebral artery. Surg Neurol. 1986. 26: 129-41
8. Heros RC, Fritsch MJ. Surgical management of middle cerebral artery aneurysms. Neurosurgery. 2001. 48: 780-5
9. Lawton MT, Spetzler RF. Surgical strategies for giant intracranial aneurysms. Neurosurg Clin N Am. 1998. 9: 725-42
10. Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001. 94: 712-7
11. Nussbaum ES, Madison MT, Goddard JK, Lassig JP, Nussbaum LA. Peripheral intracranial aneurysms: Management challenges in 60 consecutive cases. J Neurosurg. 2009. 110: 7-13
12. Ohkuma H, Suzuki S, Ogane K. Study Group of the Association of cerebrovascular disease in Tohoku, Japan. Dissecting aneurysms of intracranial carotid circulation. Stroke. 2002. 33: 941-7
13. Pia HW, Zierski J. Giant cerebral aneurysms. A review of clinical picture, diagnosis and management with illustrative cases. Neurosurg Rev. 1982. 5: 117-48
14. Pianeti G, Barros RS, Cabral G, Carneiro FD, Lauar EH. Giant medial cerebral artery aneurysm. Case report. Arq Neuropsiquiatr. 1978. 36: 155-9
15. Roccatagliata L, Guédin P, Condette-Auliac S, Gaillard S, Colas F, Boulin A. Partially thrombosed intracranial aneurysms: Symptoms, evolution, and therapeutic management. Acta Neurochir (Wien). 2010. 152: 2133-42
16. Romani R, Lehto H, Laakso A, Horcajadas A, Kivisaari R, von und zu Fraunberg M. Microsurgery for previously coiled aneurysms: Experience with 81 patients. Neurosurgery. 2011. 68: 140-53
17. Sciubba DM, Gallia GL, Recinos P, Garonzik IM, Clatterbuck RE. Intracranial aneurysm following radiation therapy during childhood for a brain tumor. Case report and review of the literature. J Neurosurg. 2006. 105: 134-9
18. Sinclair W. Dissecting aneurysm of the middle cerebral artery associated with migraine syndrome. Am J Pathol. 1953. 29: 1083-91
19. Sughrue ME, Saloner D, Rayz VL, Lawton MT. Giant intracranial aneurysms: Evolution of management in a contemporary surgical series. Neurosurgery. 2011. 69: 1261-70
20. Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: Intraoperative changes and postoperative outcome. J Neurosurg. 2006. 105: 675-81
21. van Rooij WJ, Sluzewski M. Endovascular treatment of large and giant aneurysms. AJNR Am J Neuroradiol. 2009. 30: 12-8
22. Waldron JS, Halbach VV, Lawton MT. Microsurgical management of incompletely coiled and recurrent aneurysms: Trends, techniques, and observations on coil extrusion. Neurosurgery. 2009. 64: S301-15
23. Zhu W, Liu P, Tian Y, Gu Y, Xu B, Chen L. Complex middle cerebral artery aneurysms: A new classification based on the angioarchitecture and surgical strategies. Acta Neurochir (Wien). 2013. 155: 1481-91