- Department of Neurosurgery, Treatment and Rehabilitation Center, Moscow, Russian Federation.
DOI:10.25259/SNI_56_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: George Grigoryan, Andrey Sitnikov, Yuri Grigoryan. Hemifacial spasm caused by the brainstem developmental venous anomaly: A case report and review of the literature. 06-Jun-2020;11:141
How to cite this URL: George Grigoryan, Andrey Sitnikov, Yuri Grigoryan. Hemifacial spasm caused by the brainstem developmental venous anomaly: A case report and review of the literature. 06-Jun-2020;11:141. Available from: https://surgicalneurologyint.com/surgicalint-articles/10069/
Abstract
Background: Hemifacial spasm (HFS) is usually caused by vascular compression of the root exit zone (REZ) of the facial nerve. Dual compression of the REZ by veins and arteries is also associated with HFS, but venous origin alone is rarely reported. We present a rare case of HFS caused by the brainstem developmental venous anomaly (DVA) treated with microvascular decompression (MVD).
Case Description: A 30-year-old women presented with the left-sided HFS since the age of 18 years. The brainstem DVA was diagnosed by magnetic resonance imaging (MRI) and followed by two attempts of MVD at some other clinics without any improvement. At our hospital, MVD was performed through a left retromastoid craniotomy. Intraoperatively, after detaching the strong adhesions between the cerebellar hemisphere, petrosal dura and lower cranial nerves, and removing the Teflon sponge inserted during the previous operations, the compressing large vein was found, separated from facial nerve REZ and MVD was completed. The postoperative computed tomography angiography and MRI showed the thrombosis of the main trunk of DVA and decompression of the facial nerve REZ. Complete cessation of HFS with hearing preservation was observed with only slight weakness of mimic muscles which disappeared within 3 months after surgery.
Conclusion: HFS associated with brainstem DVA is a very rare condition. MVD of the facial nerve REZ with transposition of the large draining vein should be considered as an effective treatment option.
Keywords: Brainstem, Developmental venous anomaly, Hemifacial spasm, Microvascular decompression, Venous angioma
INTRODUCTION
Hemifacial spasm (HFS) is usually caused by vascular compression of the root exit zone (REZ) of the facial nerve. The offending vessels in most cases of HFS are the anterior and posterior inferior cerebellar arteries and the tortuous vertebrobasilar artery. Dual compression of the REZ by veins and arteries is also associated with HFS, but venous origin alone is rarely reported.[
CASE DESCRIPTION
A 30-year-old otherwise healthy women presented with the left-sided HFS since the age of 18 years. She initially experienced spontaneous intermittent twitching of orbicularis oculi muscle that gradually progressed in frequency and severity with following spread to other muscles innervated by the facial nerve.
A brainstem DVA was diagnosed by magnetic resonance imaging (MRI) revealing the large venous channel near the facial nerve REZ. At the age of 26, a left retromastoid craniotomy was performed with MVD of the facial nerve without any improvement of patient’s spasm. One year later, she underwent second MVD at another clinic, but symptoms were unchanged. At admission, the patient presented with severe left clonic-tonic mimic muscles and platysma contractions exacerbated by voluntary facial movements, fatigue, and psychological stress without any acoustic phenomena or other neurological signs.
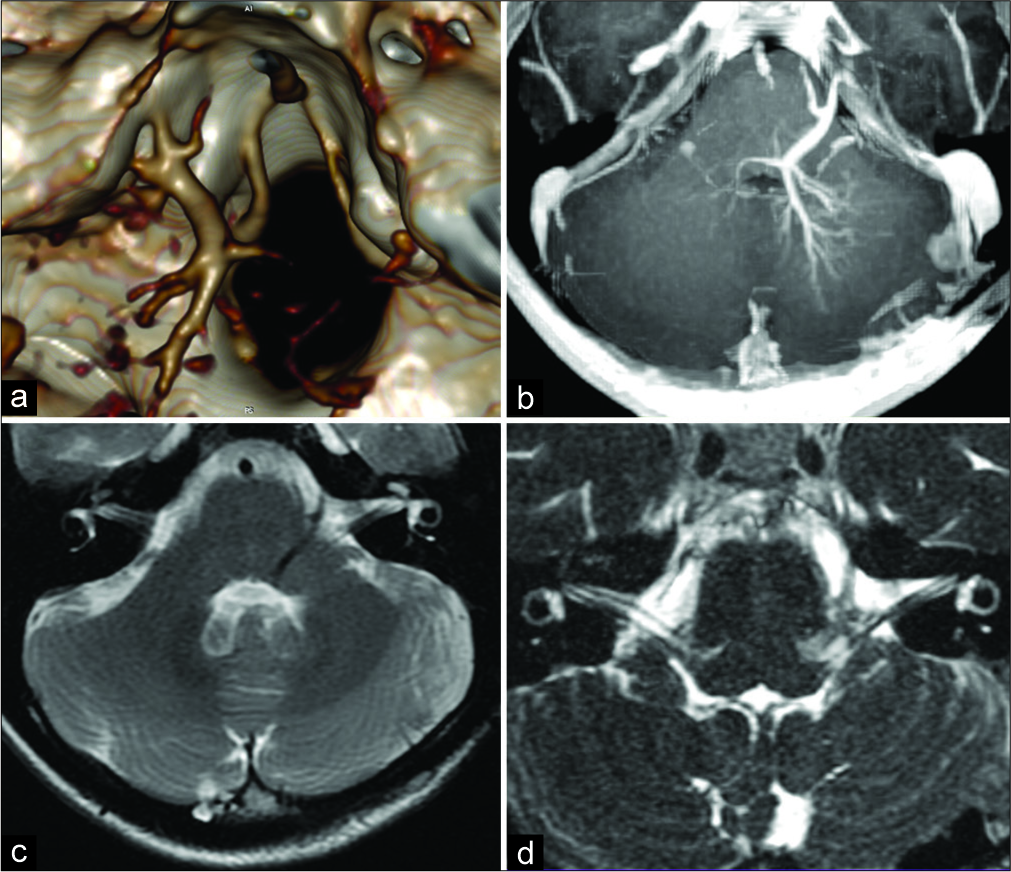
Preoperative computed tomography (CT) angiography showed the collection of abnormal dilated medullary veins in the left cerebellar peduncle and the brainstem merging into a single large vein. This collecting vein was located in the left cerebellopontine angle and drained into the inferior petrosal sinus. MRI with contrast enhancement demonstrated the typical appearance of the brainstem DVA. The small venous channels forming “caput medusae” were located in the left cerebellar hemisphere adjacent to the fourth ventricle, in the medulla oblongata and the pons. The large draining vein was passing through the brainstem near lateral recess of the fourth ventricle into the cerebellopontine cistern. The vein reached the surface of the brainstem at the root of the facial nerve [
Figure 1:
Preoperative images. (a) Three-dimensional computed tomography angiogram and (b-d) magnetic resonance imaging showing the collection of abnormal dilated medullary veins in the left cerebellar peduncle and the brainstem merging into a single large vein compressing the facial nerve root exit zone.
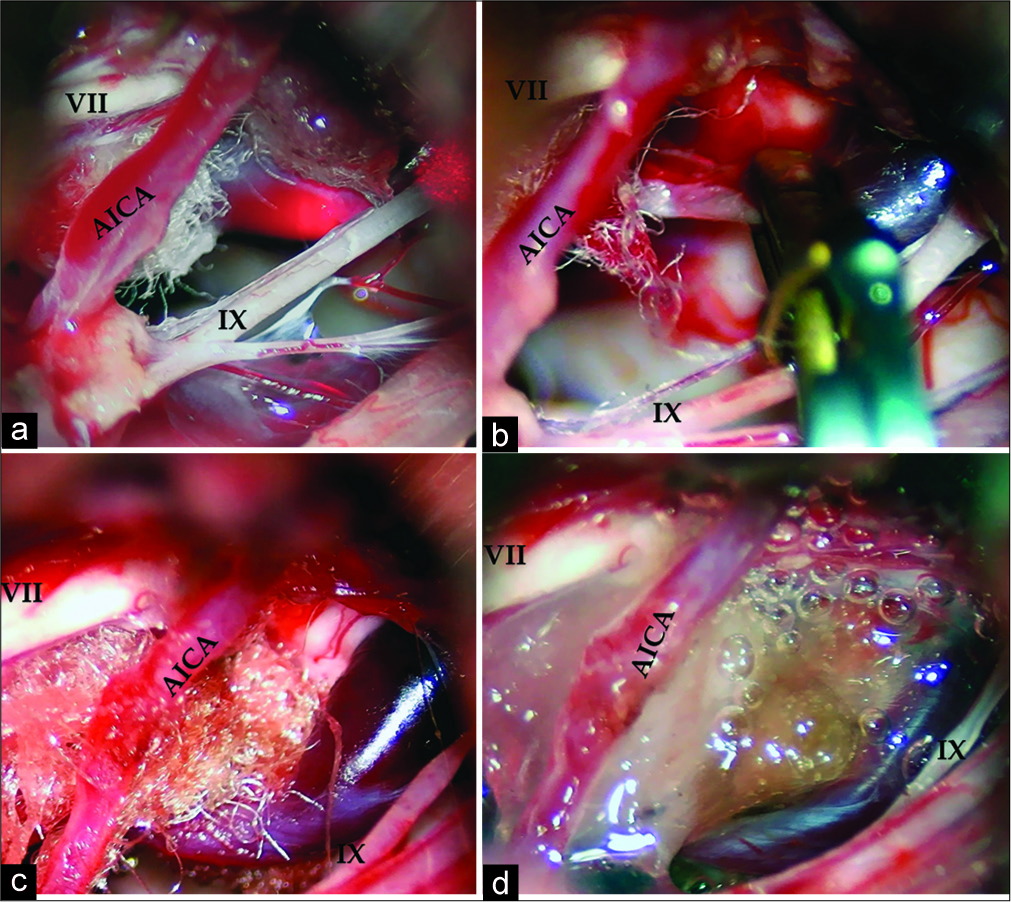
MVD was performed through a left retromastoid craniotomy, with patient in semi-sitting position. Intraoperative observation revealed strong adhesions between the cerebellar hemisphere, petrosal dura, and lower cranial nerves. After detaching of adhesions, the brainstem and the nerve roots were clearly visualized. The Teflon sponge inserted during previous operations was found under the cisternal portion of the facial and the vestibulocochlear nerves and was removed. The large vein emerged the middle cerebellar peduncle and the choroid plexus of the lateral recess of the fourth ventricle dorsal of the origin of the caudal cranial nerves and run through the cerebellopontine cistern under the facial nerve. The facial nerve REZ was cross-compressed with the main trunk of DVA strongly adherent to the pial surface of the brainstem. The small distal brunch of DVA was coagulated and cut and the main trunk was occluded near the cerebellar peduncle with temporary clip. The large vein emptied and became flaccid and the vessel was sharply dissected and carefully transposed ventrally to relieve the compression of the facial nerve REZ. The MVD was completed by insertion of Surgicel and fibrin glue between the brainstem and the displaced draining vein, and temporary clip was released [
Figure 2:
Intraoperative images. (a) The Teflon sponge inserted earlier was seen under the cisternal portion of the facial and the vestibulocochlear nerves. The large vein emerged the lateral recess of the IV ventricle run under the facial nerve root exit zone (REZ); (b) the main trunk of the brainstem developmental venous anomaly was clipped near the cerebellar peduncle with temporary clip; (c) the vein was dissected and transposed ventrally to relieve the compression of the facial nerve REZ with insertion of “Surgicel” between the brainstem and the displaced draining vein; (d) transposed large vein was fixed with fibrin glue. (VII – the facial nerve, IX – the glossopharyngeal nerve, AICA – the anterior inferior cerebellar artery).
DISCUSSION
The most common vascular anomalies of the brain are DVA, affecting 2.5–8.7% of population, and represent congenital variant of the normal venous drainage. DVA is composed of dilated medullary veins forming the so-called “caput medusae” and merge in large transcortical or subependymal collector vein. They are considered as compensatory venous pathways developing anomaly, caused by the absence of normal veins because of aplasia, hypoplasia, or occlusion during fetal life. In the posterior cranial fossa, DVA usually drains through the central pontine vein or vein of the lateral recess of the fourth ventricle and empties into petrosal sinuses. Most DVAs are asymptomatic and incidentally found during neuroimaging studies. DVA management remains controversial and generally do not require treatment. Although it is generally accepted that DVAs have a very low risk of rupture and bleeding, intracranial hemorrhage may be the present in a small percentage of patients, especially in cases with coincident adjacent cavernous angioma. Neurological manifestations can be associated with intracerebral clot formation and edema resulting from thrombosis of the DVA. Pathophysiological mechanisms underlying the clinical manifestations of symptomatic DVAs are divided into idiopathic, flow-related (misbalance of the in- and outflow of blood), and mechanical causes (direct compression of neural structures).[
Rare cases of DVA causing mechanical compression of the aqueduct with obstructive hydrocephalus were described and treated with ventriculoperitoneal shunting, aqueductal stenting, and endoscopic third ventriculoscopy.[
Venous compression of the root of the facial nerve is rare in HFS and the veins alone as offending vessels are reported in 0%–7.9%.[
There are very few cases of HFS associated with the brainstem DVA reported in literature. Chiaramonte et al. presented a man with the left-sided HFS and MRI demonstrated the typical brainstem DVA with “caput medusae” drained through dilated venous trunk into the superior petrosal sinus. The main venous collector went along the edge of the ventrolateral brainstem causing compression of the facial nerve REZ. Surgery was refused and the patient was managed with carbamazepine.[
Surgical experience gained in the brainstem DVA associated with the trigeminal neuralgia showed good long-term effectiveness of MVD. It was demonstrated that transposition of the DVAs compressing veins and offending arteries is the best optional treatment for trigeminal neuralgia. Ligation of the main trunk of the DVA in four cases was uneventful and patients became pain free, but one patient died due to cerebellar infarction. Any draining vein injury caused by the surgical procedure may result in congestive ischemic damage of the brainstem or cerebellar infarction. Therefore, vascular injuries to the main collector and tributaries of the DVA must be avoided.[
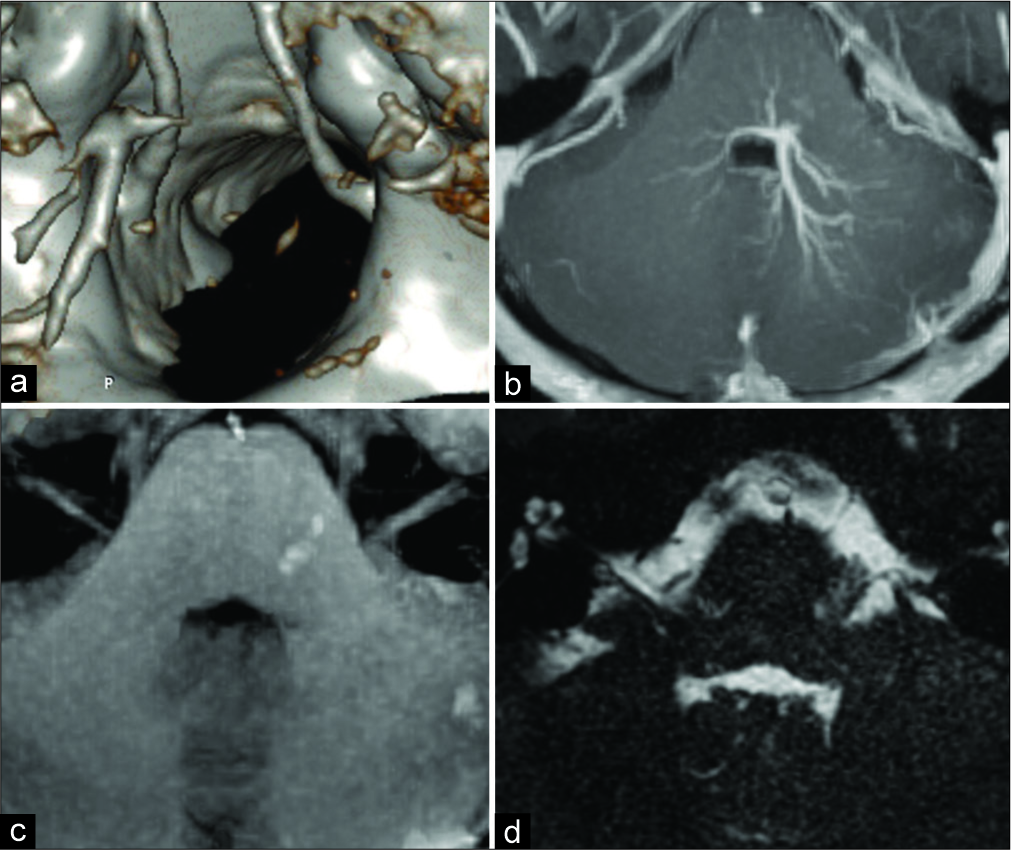
In the present case of HFS, postoperative thrombosis of the main venous collector most likely was developed because of temporary clipping and coagulation of the small branch of the brainstem DVA. These surgical manipulations were performed for transposition of the big venous channel away from the facial nerve REZ. Thrombosed draining vein was clearly demonstrated with MRI and CT and had no neurological consequences. Mobilization of the large vein strongly adherent with the pial surface of the brainstem, especially in distal parenchymatous segment of the brainstem DVA, required the use of sharp dissection resulted in damage of the facial nerve root fibers emerging from the pons. Postoperative slight facial nerve palsy was temporary with subsequent full recovery without recurrence of HFS.
CONCLUSION
HFS associated with the brainstem DVA is a very rare condition. MVD of the facial nerve REZ with transposition of the large draining vein should be considered as an effective treatment option.
Declaration of patient consent
Patient’s consent not obtained as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Acioly M, Simões E, Parise M, Telles C, Nigri F. Developmental venous anomaly causing trigeminal neuralgia. Arq Neuropsiquiatr. 2010. 68: 822-5
2. Amuluru K, Al-Mufti F, Hannaford S, Singh I, Prestigiacomo C, Gandhi C. Symptomatic infratentorial thrombosed developmental venous anomaly: Case report and review of the literature. Interv Neurol. 2015. 4: 130-7
3. Aoki R, Srivatanakul K. Developmental venous anomaly: Benign or not benign. Neurol Med Chir (Tokyo). 2016. 56: 534-43
4. Arita H, Kishima H, Hosomi K, Iwaisako K, Hashimoto N, Saitoh Y. Hemifacial spasm caused by intra-axial brainstem cavernous angioma with venous angiomas. Br J Neurosurg. 2011. 26: 281-3
5. Chen HJ, Lee TC, Lui CC. Hemifacial spasm caused by a venous angioma. J Neurosurg. 1996. 85: 716-7
6. Chiaramonte R, Bonfiglio M, D’Amore A, Chiaramonte I. Developmental venous anomaly responsible for hemifacial spasm. Neuroradiol J. 2013. 26: 201-7
7. Damiano T, Truwit C, Dowd C, Symonds D. Posterior fossa venous angiomas with drainage through the brain stem. AJNR Am J Neuroradiol. 1994. 15: 643-52
8. Eun J, Choi J, Son B. Hemifacial spasm caused by a vein: A case report. Asian J Neurosurg. 2018. 13: 786-8
9. Griffiths D, Newey A, Faulder K, Steinfort B, Krause M. Thrombosis of a developmental venous anomaly causing venous infarction and pontine hemorrhage. J Stroke Cerebrovasc Dis. 2013. 22: e653-5
10. Jannetta P. Hemifacial spasm caused by a venule: Case report. Neurosurgery. 1984. 14: 89-92
11. Kiroglu Y, Oran I, Dalbasti T, Karabulut N, Calli C. Thrombosis of a drainage vein in developmental venous anomaly (DVA) leading venous infarction: A case report and review of the literature. J Neuroimaging. 2011. 21: 197-201
12. Kita D, Park C, Hayashi Y. Aqueductal developmental venous anomaly presenting with mimic symptoms of idiopathic normal pressure hydrocephalus in an elderly patient: A case report. NMC Case Rep J. 2019. 6: 83-6
13. Korinth M, Möller-Hartmann W, Gilsbach J. Microvascular decompression of a developmental venous anomaly in the cerebellopontine angle causing trigeminal neuralgia. Br J Neurosurg. 2002. 16: 52-5
14. Küker W, Mull M, Thron A. Developmental venous anomalies of the posterior fossa with transpontine drainage: Report of 3 cases. Eur Radiol. 1997. 7: 913-7
15. Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): The so-called venous angioma. Neurosurg Rev. 1986. 9: 233-42
16. Maish W. Developmental venous anomalies and brainstem cavernous malformations: A proposed physiological mechanism for haemorrhage. Neurosurg Rev. 2018. 42: 663-70
17. Malinvaud D, Lecanu J, Halimi P, Avan P, Bonfils P. Tinnitus and cerebellar developmental venous anomaly. Arch Otolaryngol Head Neck Surg. 2006. 132: 550-3
18. McLaughlin M, Kondziolka D, Flickinger J, Lunsford S, Lunsford L. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998. 43: 195-200
19. Meng G, Bai C, Yu T, Wu Z, Liu X, Zhang J. The association between cerebral developmental venous anomaly and concomitant cavernous malformation: An observational study using magnetic resonance imaging. BMC Neurol. 2014. 14: 50-
20. Nagata K, Nikaido Y, Yuasa T, Fujioka M, Ida Y, Fujimoto K. Trigeminal neuralgia associated with venous angioma--case report. Neurol Med Chir (Tokyo). 1995. 35: 310-3
21. Nunna R, Jhaveri M, Byrne R. Trigeminal neuralgia caused by dual compressive pathology of developmental venous anomaly and small enhancing lesion. Interdiscip Neurosurg. 2018. 11: 11-3
22. Pereira V, Geibprasert S, Krings T, Aurboonyawat T, Ozanne A, Toulgoat F. Pathomechanisms of symptomatic developmental venous anomalies. Stroke. 2008. 39: 3201-15
23. Peterson A, Williams R, Fukui M, Meltzer C. Venous angioma adjacent to the root entry zone of the trigeminal nerve: Implications for management of trigeminal neuralgia. Neuroradiology. 2002. 44: 342-6
24. Rammos S, Maina R, Lanzino G. Developmental venous anomalies: Current concepts and implications for management. Neurosurgery. 2009. 65: 20-30
25. Rizek P, Kumar N, Sharma M, Jog M. Brainstem developmental venous anomaly causing hemifacial spasm-case report and review of the literature. Can J Neurol Sci. 2016. 43: 606-8
26. Ruíz DS, Yilmaz H, Gailloud P. Cerebral developmental venous anomalies: Current concepts. Ann Neurol. 2009. 66: 271-83
27. Samadian M, Bakhtevari M, Nosari M, Babadi A, Razaei O. Trigeminal neuralgia caused by venous angioma: A case report and review of the literature. World Neurosurg. 2015. 84: 860-4
28. Toda H, Iwasaki K, Yoshimoto N, Miki Y, Hashikata H, Goto M. Bridging veins and veins of the brainstem in microvascular decompression surgery for trigeminal neuralgia and hemifacial spasm. Neurosurg Focus. 2018. 45: E2-
29. Wang X, Thirumala P, Shah A, Gardner P, Habeych M, Crammond D. The role of vein in microvascular decompression for hemifacial spasm: A clinical analysis of 15 cases. Neurol Res. 2013. 35: 389-94
30. Xiong N, Zhao H. Hemifacial spasm caused by an offending vein and its treatment by vein-preserving microvascular decompression surgery: A case report. Chirurgia (Turin). 2015. 28: 27-30
31. Yamamoto T, SuzukI M, Esaki T, Nakao Y, Mori K. Trigeminal neuralgia caused by venous angioma: Case report. Neurol Med Chir (Tokyo). 2013. 53: 40-3
32. Yamgoue Tchameni Y, Messerer M, Zerlauth J, Levivier M, Daniel R. Isolated developmental venous anomaly of the pons with transpontine drainage: Case report. Clin Neuroradiol. 2013. 24: 77-81
33. Yang W, Kuroi Y, Yokosako S, Ohbuchi H, Tani S, Kasuya H. Hemifacial spasm caused by veins confirmed by intraoperative monitoring of abnormal muscle response. World Neurosurg X. 2019. 1: 100002-