- Department of Neurosurgery Yokohama Sakae Kyosai Hospital, Yokohama, Kanagawa, Japan.
- Department of Neurology, Yokohama Sakae Kyosai Hospital, Yokohama, Kanagawa, Japan.
- Department of Neurosurgery, Taguchi Neurosurgery Clinic, Yokohama, Kanagawa, Japan.
Correspondence Address:
Yu Iida
Department of Neurosurgery Yokohama Sakae Kyosai Hospital, Yokohama, Kanagawa, Japan.
DOI:10.25259/SNI_564_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yu Iida1, Kentaro Mori1, Yosuke Kawahara1, Issei Fukui1, Katsuya Abe2, Mutsuki Takeda2, Tastu Nakano2, Hiroki Taguchi3, Motohiro Nomura1. Hemifacial spasm caused by vertebral artery aneurysm treated by endovascular coil embolization. 11-Dec-2020;11:431
How to cite this URL: Yu Iida1, Kentaro Mori1, Yosuke Kawahara1, Issei Fukui1, Katsuya Abe2, Mutsuki Takeda2, Tastu Nakano2, Hiroki Taguchi3, Motohiro Nomura1. Hemifacial spasm caused by vertebral artery aneurysm treated by endovascular coil embolization. 11-Dec-2020;11:431. Available from: https://surgicalneurologyint.com/surgicalint-articles/10445/
Abstract
Background: Hemifacial spasm (HFS) caused by vertebral artery (VA) aneurysms is rare. Several cases of HFS caused by VA aneurysms treated by endovascular parent artery occlusion (PAO) have been reported. Recently, we treated a rare case of HFS caused by a saccular VA aneurysm at the bifurcation of the posterior inferior cerebellar artery (PICA), which was successfully treated by endovascular coil embolization, preserving the parent artery, and PICA. We discuss endovascular treatment for HFS induced by VA aneurysms with a literature review.
Case Description: A 59-year-old man presented with the left HFS persisting for 2 months. Magnetic resonance imaging revealed a left saccular VA-PICA aneurysm and demonstrated that a left facial nerve was compressed by the aneurysm at the root exit zone. Angiography revealed that the PICA was branching from the aneurysm neck. Endovascular coil embolization was performed using the balloon remodeling technique to preserve the left VA and PICA. HFS disappeared after treatment.
Conclusion: Although microvascular decompression was commonly accepted for the standard treatment of HFS, coil embolization of aneurysms without PAO may be an effective treatment for HFS caused by VA aneurysms.
Keywords: Aneurysm, Coil embolization, Endovascular treatment, Hemifacial spasm, Vertebral artery
INTRODUCTION
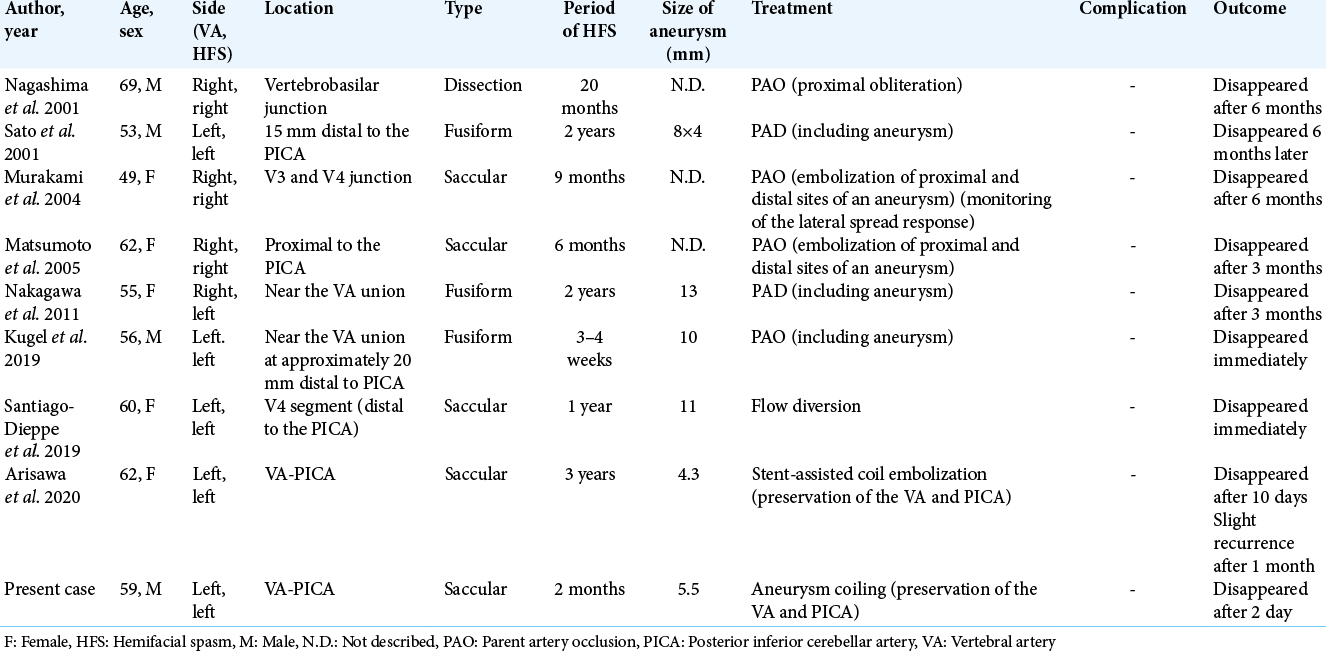
Hemifacial spasm (HFS) is usually caused by compression of the facial nerve at the root exit zone (REZ) with branches of the vertebrobasilar system or tortuously elongated vertebral artery (VA) itself. On the other hand, a VA aneurysm is a rare cause of HFS. Eight cases of HFS caused by VA aneurysms treated by endovascular therapy have been reported in English.[
CASE DESCRIPTION
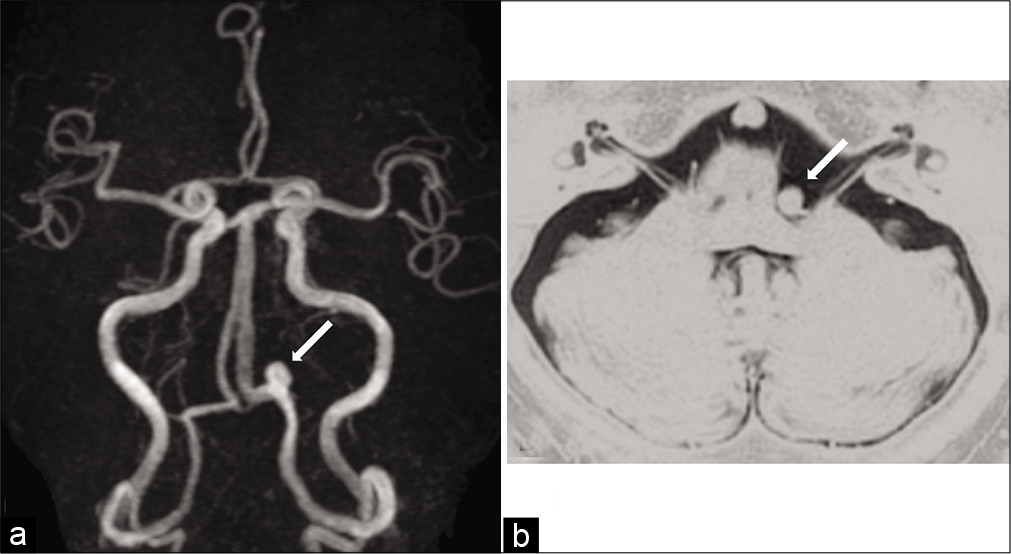
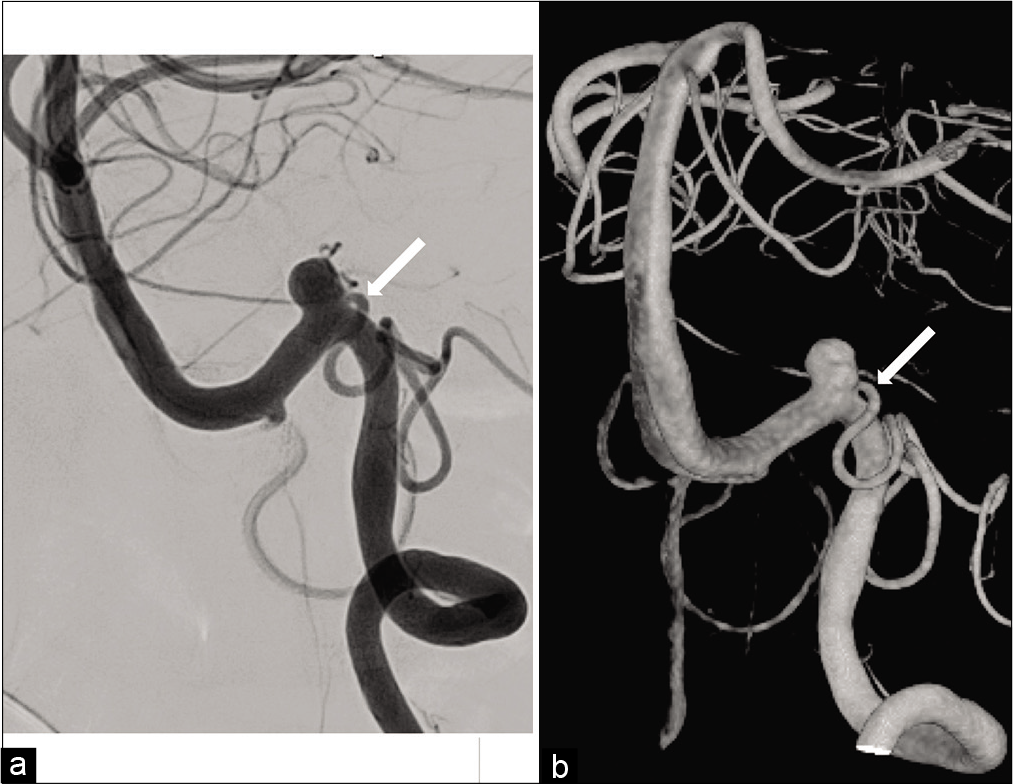
A 59-year-old man with hypertension and diabetes mellitus was referred to our hospital with a 2-month history of the left HFS. The patient had no neurological deficit except for left HFS. There was no episode of severe occipitalgia to suggest VA dissection. Magnetic resonance angiography (MRA) demonstrated a left saccular VA aneurysm, and the left VA was dominant [
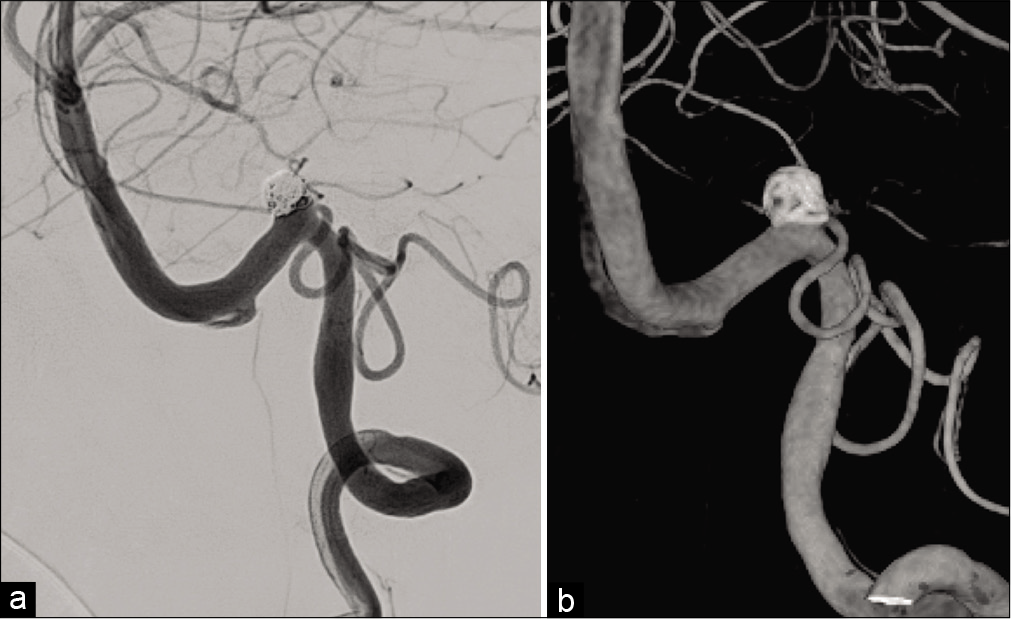
Antiplatelet drug administration of aspirin at 100 mg/day and clopidogrel at 75 mg/day was started 1 week before treatment. Procedures were performed under general anesthesia. An 8-Fr. long sheath introducer was inserted into the right femoral artery. Systemic heparinization was introduced and the activated clotting time was maintained at 200–250 s. A 6-Fr. guiding catheter (FUBUKI, Asahi Intecc, Aichi, Japan) was guided into the left VA. Coil embolization was performed by the balloon-assisted technique to preserve the left PICA and VA. A balloon catheter (Scepter XC, 4 × 11 mm, Terumo, Tokyo, Japan) was advanced into the VA distal to the neck of the aneurysm, and a microcatheter (Excelsior SL-10, Stryker, Kalamazoo, MI, USA) was inserted into the aneurysm using a microguidewire (CHIKAI 14, Asahi Intecc). A platinum coil (Target 360 soft, 5 mm × 10 cm, Stryker) as the first coil was deployed in the aneurysm after the micro-balloon was placed at the aneurysm neck and inflated. A total of 12 coils were inserted serially, and the aneurysm was obliterated while preserving the PICA and parent artery [
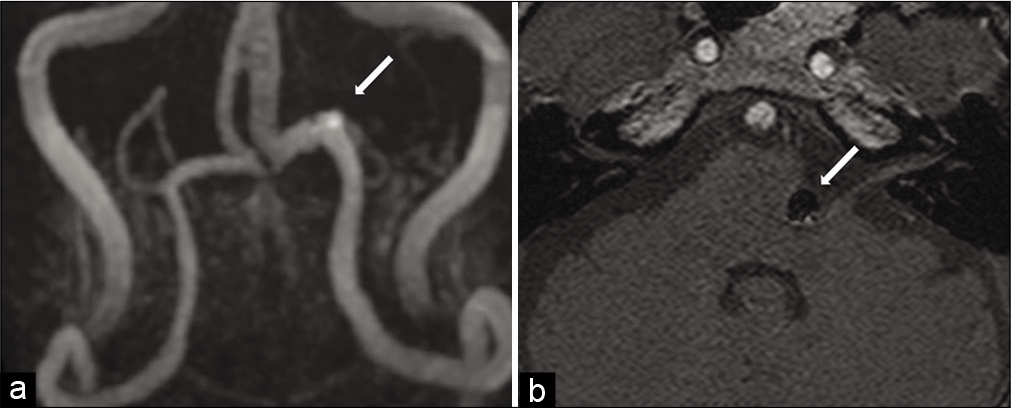
His postoperative course was uneventful. No new neurological symptoms developed, and ischemic lesions were not identified on diffusion-weighted MRI. The spasm of the left eyelid muscle persisted on the day of surgery. The frequency of HFS decreased the next day after treatment, and HFS disappeared 2 days later. Postoperative MRA demonstrated no blood flow within the aneurysm [
DISCUSSION
HFS is usually caused by compression of the facial nerve REZ where the central and peripheral myelin connects. The cause of compression is generally vessels, and occasionally tumors,[
HFS commonly disappears immediately after direct surgery, such as MVD,[
In our case, reduction of compression was not achieved, but HFS disappeared after coil embolization. It was suggested that pulsation rather than compression has more influence on HFS. In cases of ONP, Guresir et al. reported that the rate of complete ONP resolution did not differ between patients undergoing simple clipping and those who underwent clipping with nerve decompression, and the resolution of ONP is inversely correlated with the initial severity of ONP.[
Santiago-Dieppa et al. reported a case of endovascular flow diversion for a VA aneurysm inducing HFS.[
CONCLUSION
HFS caused by VA aneurysms is rare. Although coil embolization of the aneurysm was unable to reduce compression, HFS caused by the VA-PICA aneurysm improved after endovascular treatment. HFS was suggested to be mainly induced by pulsation of a VA aneurysm rather than direct compression of the REZ, and coil embolization of such aneurysms without PAO may be an effective treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arisawa K, Ochi T, Goto Y, Nanbu S, Shojima M, Maeda K. Coil embolization of VA-PICA aneurysm presenting with hemifacial spasm with assistance of abnormal muscle response monitoring. J Neuroendovasc Ther. 2020. 14: 146-50
2. Asaoka K, Sawamura Y, Tada M, Abe H. Hemifacial spasm caused by a hemangioma at the geniculate ganglion: Case report. Neurosurgery. 1997. 41: 1195-7
3. Choi SK, Rhee BA, Park BJ, Lim YJ. Hemifacial spasm caused by fusiform aneurysm at vertebral artery-posterior inferior cerebellar artery junction. J Korean Neurosurg Soc. 2008. 44: 399-400
4. Furtado SV, Thakar S, Saikiran NA, Hegde AS. Hemifacial spasm and jugular foramen syndrome caused by diametrically opposite aneurysms on the vertebral artery. Neurol Sci. 2013. 34: 1809-10
5. Gu DQ, Luo B, Zhang X, Long XA, Duan CZ. Recovery of posterior communicating artery aneurysm-induced oculomotor nerve paresis after endovascular treatment. Clin Neurol Neurosurg. 2012. 114: 1238-42
6. Guresir E, Schuss P, Seifert V, Vatter H. Oculomotor nerve palsy by posterior communicating artery aneurysms: Influence of surgical strategy on recovery. J Neurosurg. 2012. 117: 904-10
7. Hassan T, Hamimi A. Successful endovascular management of brain aneurysms presenting with mass effect and cranial nerve palsy. Neurosurg Rev. 2013. 36: 87-97
8. Konan AV, Roy D, Raymond J. Endovascular treatment of hemifacial spasm associated with a cerebral arteriovenous malformation using transvenous embolization: Case report. Neurosurgery. 1999. 44: 663-6
9. Kudo A, Suzuki M, Kubo N, Kuroda K, Ogawa A, Iwasaki Y. Schwannoma arising from the intermediate nerve and manifesting as hemifacial spasm. Case report. J Neurosurg. 1996. 84: 277-9
10. Kugai M, Suyama T, Inui T, Yamazato K, Kitano M, Hasegawa H. A case of vertebral artery aneurysm causing hemifacial spasm rapidly improved after parent artery occlusion. J Neuroendovasc Ther. 2019. 13: 288-92
11. Matsumoto K, Kimura S, Kakita K. Endovascular treatment of vertebral artery aneurysm manifesting as progressive hemifacial spasm. Neurol Med Chir (Tokyo). 2005. 45: 360-2
12. Matsumoto K, Saijo T, Kuyama H, Asari S, Nishimoto A. Hemifacial spasm caused by a spontaneous dissecting aneurysm of the vertebral artery. Case report. J Neurosurg. 1991. 74: 650-2
13. Moriuchi S, Nakagawa H, Yamada M, Kadota T. Hemifacial spasm due to compression of the facial nerve by vertebral artery-posterior inferior cerebellar artery aneurysm and elongated vertebral artery-case report. Neurol Med Chir (Tokyo). 1996. 36: 884-7
14. Murakami H, Kawaguchi T, Fukuda M, Ito Y, Hasegawa H, Tanaka R. Monitoring of the lateral spread response in the endovascular treatment of a hemifacial spasm caused by an unruptured vertebral artery aneurysm. Case report. J Neurosurg. 2004. 101: 861-3
15. Nagashima H, Orz Y, Okudera H, Kobayashi S, Ichinose Y. Remission of hemifacial spasm after proximal occlusion of vertebrobasilar dissecting aneurysm with coils: Case report. J Clin Neurosci. 2001. 8: 43-5
16. Nakagawa I, Takayama K, Kurokawa S, Wada T, Nakagawa H, Kichikawa K. Hemifacial spasm due to contralateral aneurysmal compression of the facial nerve successfully treated with endovascular coil embolization: Case report. Neurosurgery. 2011. 69: E768-71
17. Ou C, Wang S, Chen Y, Mo J, Zhao X. Microvascular decompression for hemifacial spasm induced by vertebral artery dissecting aneurysm: One case report. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016. 45: 536-9
18. Rodriguez-Catarino M, Frisen L, Wikholm G, Elfverson J, Quiding L, Svendsen P. Internal carotid artery aneurysms, cranial nerve dysfunction and headache: The role of deformation and pulsation. Neuroradiology. 2003. 45: 236-40
19. Santiago-Dieppa DR, McDonald MA, Brandel MG, Rennert RC, Khalessi AA, Olson SE. Endovascular flow diversion for hemifacial spasm induced by a vertebral artery aneurysm: First experience. Oper Neurosurg (Hagerstown). 2019. 17: E115-8
20. Sato K, Ezura M, Takahashi A, Yoshimoto T. Fusiform aneurysm of the vertebral artery presenting hemifacial spasm treated by intravascular embolization: Case report. Surg Neurol. 2001. 56: 52-5
21. Suzuki T, Takao H, Suzuki T, Kambayashi Y, Watanabe M, Shinohara S. Fluid structure interaction analysis reveals facial nerve palsy caused by vertebral-posterior inferior cerebellar artery aneurysm. Comput Biol Med. 2015. 66: 263-8
22. Takahara M, Abe H, Ohkawa M, Iwaasa M, Ueba T, Higashi T. Hemifacial spasm caused by a dissecting aneurysm of the vertebral artery, and resulting in acute exacerbation. No Shinkei Geka. 2013. 41: 241-6
23. Tsuchiya D, Kayama T, Saito S, Sato S. Hemifacial spasm due to a compression of the facial nerve by a fusiform aneurysm of the vertebral artery: Case report. No To Shinkei. 2000. 52: 517-21
24. Uchino M, Nomoto J, Ohtsuka T, Kuramitsu T. Fusiform aneurysm of the vertebral artery presenting with hemifacial spasm treated by microvascular decompression. Acta Neurochir (Wien). 2005. 147: 901-3