- Department of Neurosurgery, National Neuroscience Institute, King Fahad Medical City, Riyadh, Saudi Arabia,
- College of Medicine, King Saud University, Riyadh, Saudi Arabia,
- Department of Pathology and Clinical Laboratory Medicine, King Fahad Medical City, Riyadh, Saudi Arabia.
Correspondence Address:
Gmaan Alzhrani
Department of Neurosurgery, National Neuroscience Institute, King Fahad Medical City, Riyadh, Saudi Arabia,
DOI:10.25259/SNI_583_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sarah Bin Abdulqader1, Nasser Almujaiwel2, Wafa Alshakweer3, Gmaan Alzhrani1. High-grade spheno-orbital meningioma in patients with systemic lupus erythematosus: Two case reports and literature review. 29-Oct-2020;11:367
How to cite this URL: Sarah Bin Abdulqader1, Nasser Almujaiwel2, Wafa Alshakweer3, Gmaan Alzhrani1. High-grade spheno-orbital meningioma in patients with systemic lupus erythematosus: Two case reports and literature review. 29-Oct-2020;11:367. Available from: https://surgicalneurologyint.com/surgicalint-articles/10355/
Abstract
Background: Spheno-orbital meningiomas (SOMs) are often benign. The association of meningioma and systemic lupus erythematosus (SLE) is rarely discussed in the literature. Here, we report two patients with high-grade, SOMs with a prolonged history of SLE and review the literature.
Case Description: The first case is a 52-year-old female patient with a 15-year history of SLE diagnosis who was referred to our center with a 1-year history of proptosis and excessive tearing of the left eye. This patient was operated for the left SOM with histopathological diagnosis of the World Health Organization (WHO) Grade III rhabdoid meningioma. The second case is a 36-year-old female patient with a 12-year history of SLE diagnosis who presented to our clinic with a 5-year-history of progressive right eye proptosis and occasional headaches. She was operated for the right SOM with histopathological diagnosis of the WHO Grade II chordoid meningioma.
Conclusion: Rhabdoid and chordoid SOMs are uncommon and no previous report discussed their occurrence in patients with SLE. The association of high-grade meningiomas and SLE deserves further exploration.
Keywords: Chordoid meningioma, Rhabdoid meningioma, Spheno-orbital meningioma, Systemic lupus erythematosus
INTRODUCTION
Spheno-orbital meningioma (SOM) is a complex and unique pathological condition that accounts for 9% of all intracranial meningiomas.[
Systemic lupus erythematosus (SLE) is an autoimmune, multiorgan, connective tissue disease with diverse pathogenesis and unexplored etiology that frequently affects women.[
In this paper, we describe two patients who were diagnosed with SLE and referred to us for the surgical treatment of SOM. These patients exhibited uncommon histopathological variants of SOM.
CASE REPORT
Case 1
A 52-year-old female patient with a 15-year history of SLE diagnosis was referred to our center with a 1-year history of proptosis and excessive tearing of the left eye. On examination, she was found to have a visual acuity of 20/20 in the right eye and 20/25 in the left eye, with intact extraocular movement and facial sensation. She had been on a regimen of azathioprine therapy to manage her SLE.
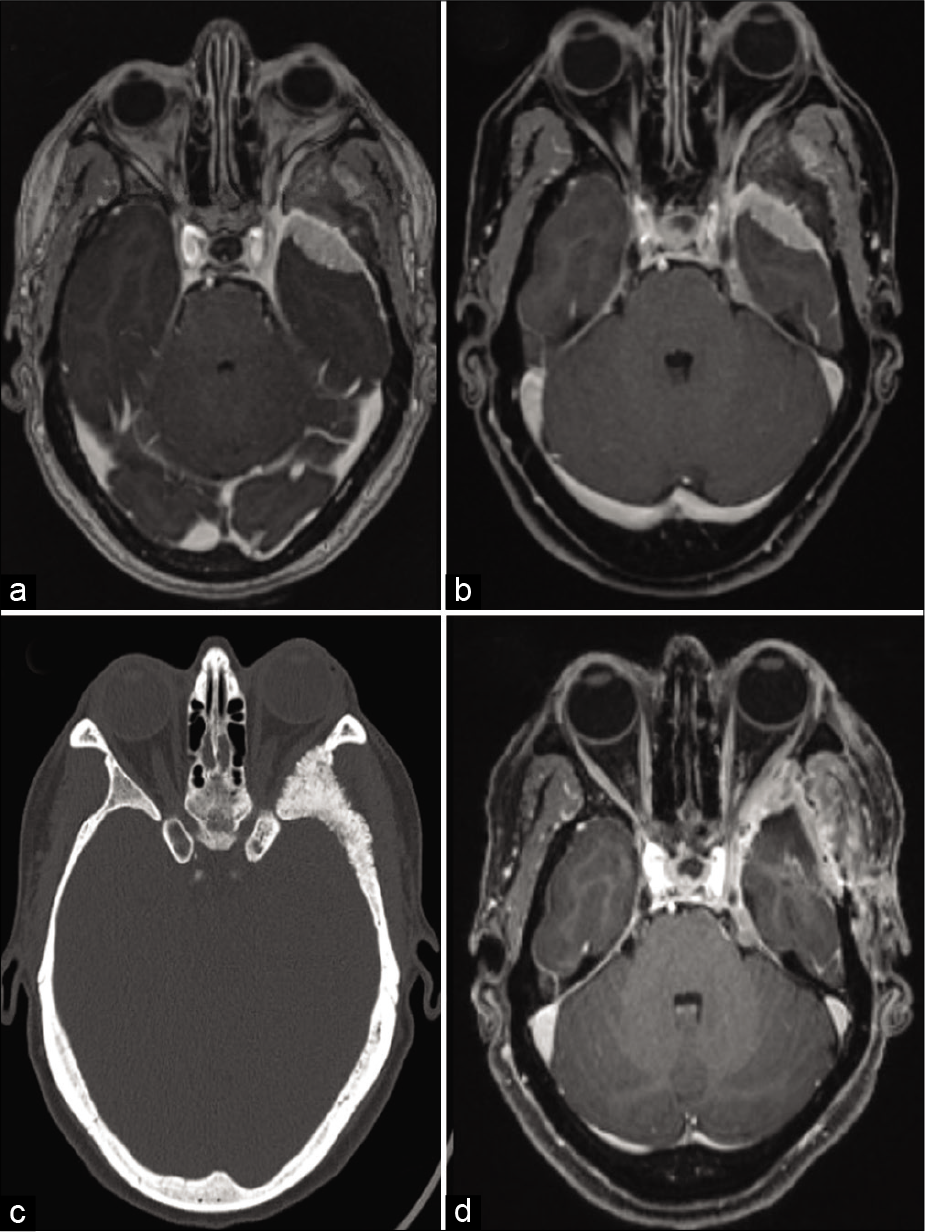
Radiological imaging showed left SOM with extension into the superior orbital fissure, left cavernous sinus, and left petrous apex. In addition, there was a small extension toward the left cerebellopontine angle (CPA) with significant hyperostosis of the sphenoid bone and lateral orbital wall [
Figure 1:
Case 1. (a and b) Preoperative T1-weighted postgadolinium administration MR images demonstrating left SOM with extension into the superior orbital fissure, left cavernous sinus, left petrous apex, and left cerebellopontine angle (CPA). (c) Preoperative CT head bone window demonstrating hyperostosis. (d) Postoperative T1-weighted postgadolinium administration MR images demonstrating postoperative changes as well as residual lesion at the left cavernous sinus and CPA.
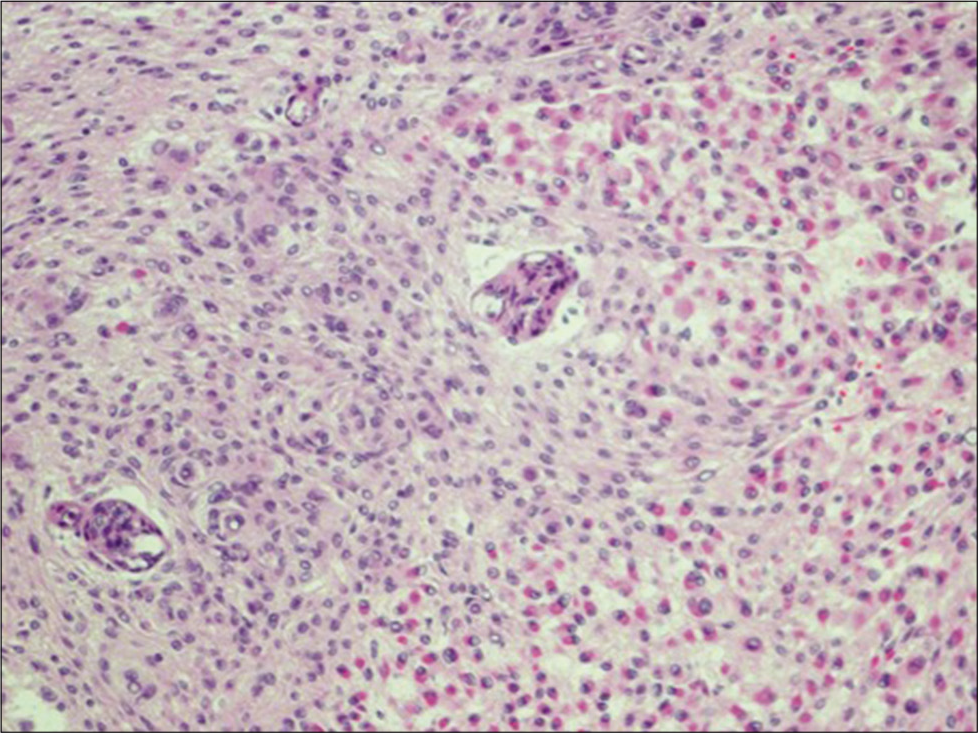
The patient was subsequently operated through the left pterional approach. The histopathological examination established a diagnosis of the World Health Organization (WHO) Grade III rhabdoid meningioma (RM) [
Case 2
A 36-year-old female patient with a 12-year history of SLE diagnosis presented to our clinic with a 5-year history of progressive right eye proptosis and occasional headaches. On examination, she was found to have right eye exophthalmos with normal visual acuity, as well as intact extraocular motility and normal facial sensation bilaterally. She was on a regimen of hydroxychloroquine to manage her lupus. In addition, she used steroids, which was discontinued a year ago because of remission.
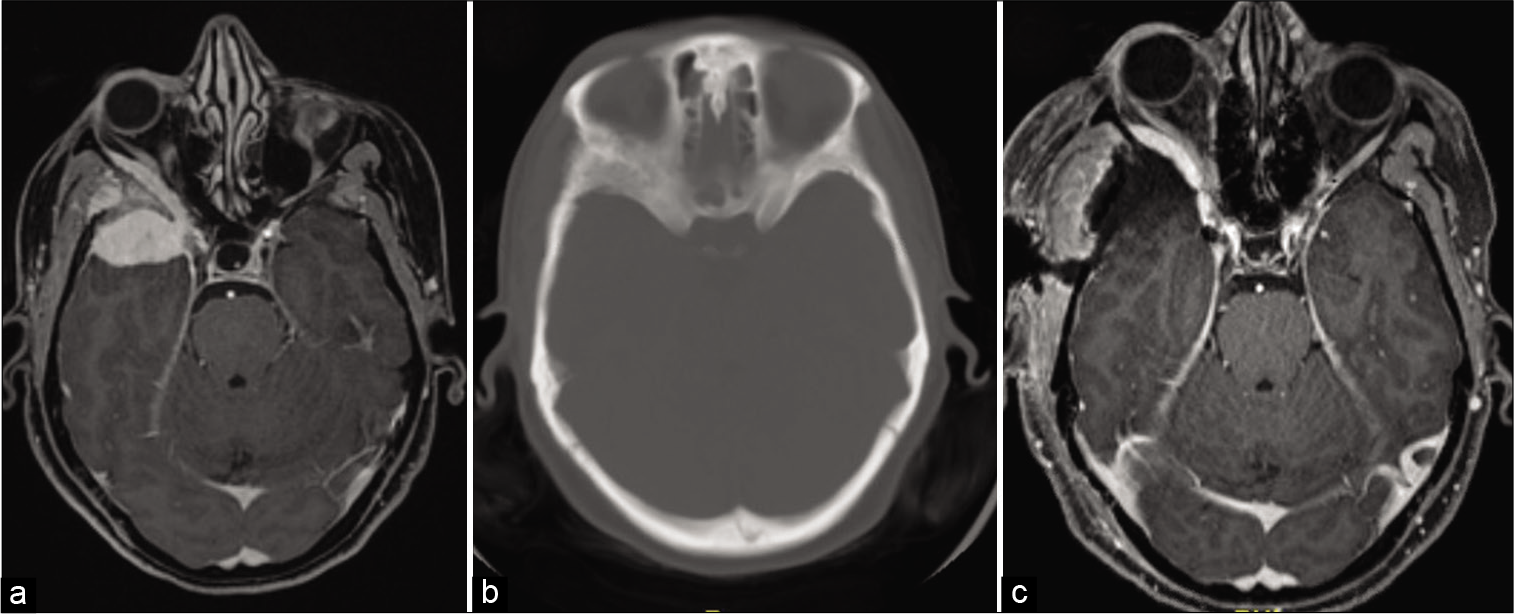
Radiological imaging findings showed right SOM with extension into the orbit and cavernous sinus [
Figure 3:
Case 2. (a) Preoperative T1-weighted postgadolinium administration MR images demonstrating right SOM extending into the right orbit and cavernous sinus. (b) Preoperative CT head demonstrating hyperostosis. (c) Postoperative T1-weighted postgadolinium administration MR images demonstrating intraorbital residual lesion approaching the superior orbital fissure.
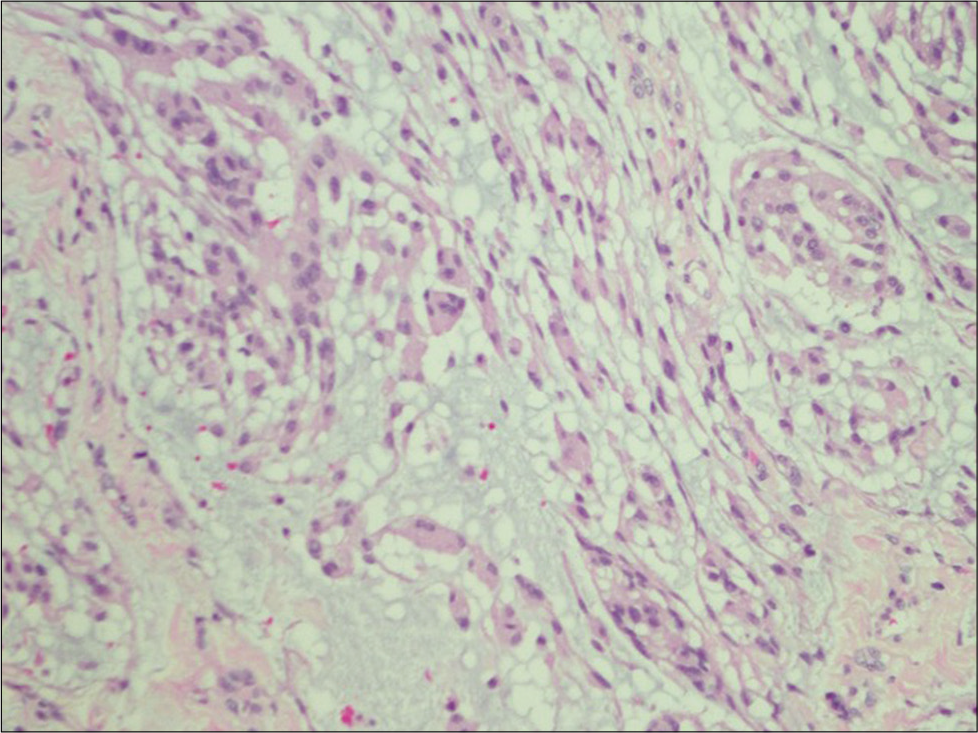
Right frontotemporal craniotomy was performed for tumor resection. The histopathological examination established a diagnosis of the WHO Grade II chordoid meningioma (CM) [
Literature review methods
To investigate for previous reports of cases of meningioma occurring in patients with SLE, we searched the PubMed database using the following terms; meningioma AND “systemic lupus erythematosus,” meningioma AND “autoimmune disease,” and meningioma AND “connective tissue disease.” Data on age, sex, time of meningioma diagnosis, location of meningioma, histopathological grade of meningioma, and medications used for the treatment of SLE were collected for each case.
Literature review results
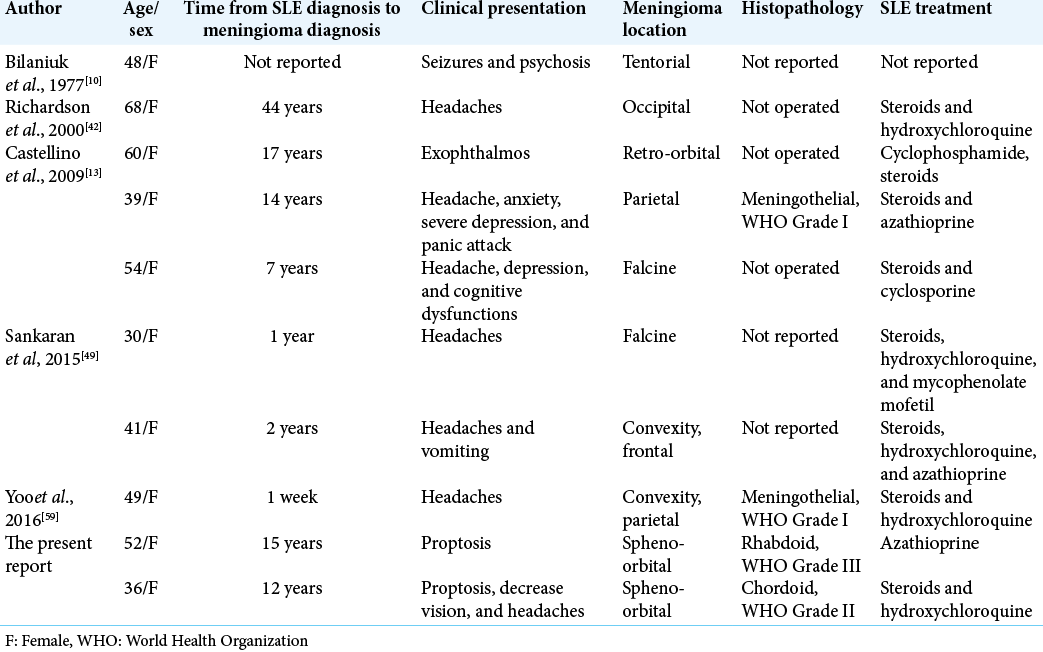
Seven cases were identified and were available for review in addition to one case that was retrieved through cited references. Ten cases were analyzed [including our two cases;
DISCUSSION
This report describes two rare histopathological variants of SOM that occurred in patients with a prolonged history of SLE. The association of meningioma and SLE has been discussed earlier;[
Meningioma is the most common primary intracranial tumor in adults, accounting for one-third of all primary intracranial tumors, with a female-to-male ratio of 2:1; and most patients have low-grade meningioma.[
RM is an uncommon variant of meningioma, which represents 0.28% of all meningioma;[
CM was additionally introduced by Kepes et al.[
Meningiomas of the cranial base show an indolent growth pattern and are often classified as low-grade meningiomas as compared with its noncranial base counterparts. The low-grade nature of these meningiomas has been theoretically attributed to the fact that most cranial base meningiomas present earlier; thus, cases of aggressive or malignant transformation are less likely to occur due to early intervention.[
High-grade SOMs, specifically RMs and CMs, are rare. Various case series have discussed the natural history and outcomes of SOMs and demonstrated that Grade I meningiomas occur in 78%,[
An association between SOM and other medical conditions, specifically, hypothyroidism has been identified;[
Prevailing theories have proposed an association between malignancies and SLE, including relatively high expression of interleukin 6 (IL-6) and IL-10 in patients with non-Hodgkin lymphoma and in patients with SLE.[
The role of estrogen and progesterone receptors in the development of meningioma is well established. This was evident by accelerated growth of meningiomas during pregnancy and in postmenopausal women receiving exogenous hormone therapy.[
From the current literature, we identified eight cases of meningiomas that were reported in patients with SLE. [
CONCLUSION
This report provides an insight into the possible attribution of SLE to the development of high-grade meningiomas. We report an unusual association between rare histopathological entities of SOM and SLE. The use of specific medications to manage SLE as well as the overexpression of IL-6 observed in both CM and SLE might play a role in the pathogenesis of high-grade (rhabdoid and chordoid) SOM. However, further epidemiological and genetic studies are needed to validate this association. Moreover, a coincidental association cannot be ruled out given that both meningioma and SLE are common disease conditions.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arima T, Natsume A, Hatano H, Nakahara N, Fujita M, Ishii D. Intraventricular chordoid meningioma presenting with Castleman disease due to overproduction of interleukin-6. Case report. J Neurosurg. 2005. 102: 733-7
2. Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012. 5: 231-42
3. Bae EH, Lim SY, Han KD, Jung JH, Choi HS, Kim CS. Systemic lupus erythematosus is a risk factor for cancer: A nationwide population-based study in Korea. Lupus. 2019. 28: 317-23
4. Baker GL, Kahl LE, Zee BC, Stolzer BL, Agarwal AK, Medsger TA. Malignancy following treatment of rheumatoid arthritis with cyclophosphamide, Long-term case-control follow-up study. Am J Med. 1987. 83: 1-9
5. Belinsky I, Murchison AP, Evans JJ, Andrews DW, Farrell CJ, Casey JP. Spheno-orbital meningiomas: An analysis based on World health organization classification and Ki-67 proliferative index. Ophthalmic Plast Reconstr Surg. 2018. 34: 143-50
6. Benson VS, Kirichek O, Beral V, Green J. Menopausal hormone therapy and central nervous system tumor risk: Large UK prospective study and meta-analysis. Int J Cancer. 2015. 136: 2369-77
7. Bernatsky S, Ramsey-Goldman R, Labrecque J, Joseph L, Boivin JF, Petri M. Cancer risk in systemic lupus: An updated international multi-centre cohort study. J Autoimmun. 2013. 42: 130-5
8. Bernatsky S, Ramsey-Goldman R, Petri M, Urowitz MB, Gladman DD, Fortin PR. Smoking is the most significant modifiable lung cancer risk factor in systemic lupus erythematosus. J Rheumatol. 2018. 45: 393-6
9. Bikmaz K, Mrak R, Al-Mefty O. Management of bone-invasive, hyperostotic sphenoid wing meningiomas. J Neurosurg. 2007. 107: 905-12
10. Bilaniuk LT, Patel S, Zimmerman RA. Computed tomography of systemic lupus erythematosus. Radiology. 1977. 124: 119-21
11. Boari N, Gagliardi F, Spina A, Bailo M, Franzin A, Mortini P. Management of spheno-orbital en plaque meningiomas: Clinical outcome in a consecutive series of 40 patients. Br J Neurosurg. 2013. 27: 84-90
12. Brenner AV, Linet MS, Fine HA, Shapiro WR, Selker RG, Black PM. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002. 99: 252-9
13. Castellino G, Rizzo N, Bernardi S, Trotta F, Govoni M. Meningioma and systemic lupus erythematosus: A matter of pure coincidence?. Lupus. 2009. 18: 650-4
14. Chen ZX, Peng XT, Tan L, Zhai GQ, Chen G, Gan TQ. EBV as a potential risk factor for hepatobiliary system cancer: A meta-analysis with 918 cases. Pathol Res Pract. 2019. 215: 278-85
15. Claus EB, Calvocoressi L, Bondy ML, Wrensch M, Wiemels JL, Schildkraut JM. Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg. 2013. 118: 649-56
16. Combs SE, Schulz-Ertner D, Debus J, von Deimling A, Hartmann C. Improved correlation of the neuropathologic classification according to adapted world health organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol Biol Phys. 2011. 81: 1415-21
17. Cophignon J, Lucena J, Clay C, Marchac D. Limits to radical treatment of spheno-orbital meningiomas. Acta Neurochir Suppl (Wien). 1979. 28: 375-80
18. Couce M, Aker F, Scheithauer B. Chordoid meningioma: A clinicopathologic study of 42 cases. Am J Surg Pathol. 2000. 24: 899-905
19. Donato G, Ferraro G, Signorelli F, Iofrida G, Lavano A, Amorosi A. Chordoid meningioma: Case report and literature review. Ultrastruct Pathol. 2006. 30: 309-14
20. Drehmer M, Andrade D, Pereira I, Marrero A, Muniz Y, de Souza I. Estrogen receptor alpha gene (ESR1) polymorphism can contribute to clinical findings in systemic lupus erythematosus patients. Lupus. 2017. 26: 294-8
21. Felten R, Scher F, Sibilia J, Chasset F, Arnaud L. Advances in the treatment of systemic lupus erythematosus: From back to the future, to the future and beyond. Joint Bone Spine. 2019. 86: 429-36
22. Forster MT, Daneshvar K, Senft C, Seifert V, Marquardt G. Sphenoorbital meningiomas: Surgical management and outcome. Neurol Res. 2014. 36: 695-700
23. Freeman JL, Davern MS, Oushy S, Sillau S, Ormond DR, Youssef AS. Spheno-orbital meningiomas: A 16-year surgical experience. World Neurosurg. 2017. 99: 369-80
24. Heufelder MJ, Sterker I, Trantakis C, Schneider JP, Meixensberger J, Hemprich A. Reconstructive and ophthalmologic outcomes following resection of spheno-orbital meningiomas. Ophthalmic Plast Reconstr Surg. 2009. 25: 223-6
25. Honig S, Trantakis C, Frerich B, Sterker I, Kortmann RD, Meixensberger J. Meningiomas involving the sphenoid wing outcome after microsurgical treatment-a clinical review of 73 cases. Cent Eur Neurosurg. 2010. 71: 189-98
26. Honig S, Trantakis C, Frerich B, Sterker I, Schober R, Meixensberger J. Spheno-orbital meningiomas: Outcome after microsurgical treatment: A clinical review of 30 cases. Neurol Res. 2010. 32: 314-25
27. Kepes JJ, Chen WY, Connors MH, Vogel FS. Chordoid meningeal tumors in young individuals with peritumoral lymphoplasmacellular infiltrates causing systemic manifestations of the Castleman syndrome. A report of seven cases. Cancer. 1988. 62: 391-406
28. Kepes JJ, Moral LA, Wilkinson SB, Abdullah A, Llena JF. Rhabdoid transformation of tumor cells in meningiomas: A histologic indication of increased proliferative activity: Report of four cases. Am J Surg Pathol. 1998. 22: 231-8
29. Kim E, Weon YC, Kim S, Kim HJ, Byun H, Lee JI. Rhabdoid meningioma: Clinical features and MR imaging findings in 15 patients. Am J Neuroradiol. 2007. 28: 1462-5
30. Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002. 61: 215-25
31. Ladouceur A, Clarke AE, Ramsey-Goldman R, Bernatsky S. Malignancies in systemic lupus erythematosus: An update. Curr Opin Rheumatol. 2019. 31: 678-81
32. Matteson EL, Hickey A, Maguire L, Tilson H, Urowitz M. Occurrence of neoplasia in patients with rheumatoid arthritis enrolled in a DMARD registry. Rheumatoid arthritis azathioprine registry steering committee. J Rheumatol. 1991. 18: 809-14
33. Menon S, Sandesh O, Anand D, Menon G. Spheno-orbital meningiomas: Optimizing visual outcome. J Neurosci Rural Pract. 2020. 11: 385-94
34. Mourits MP, van der Sprenkel JW. Orbital meningioma, the utrecht experience. Orbit. 2001. 20: 25-33
35. Nagahama A, Goto T, Nagm A, Tanoue Y, Watanabe Y, Arima H. Spheno-orbital meningioma: Surgical outcomes and management of recurrence. World Neurosurg. 2019. 126: e679-87
36. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C. CBTRUS Statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015. 17: iv1-62
37. Perry A, Scheithauer BW, Stafford SL, Abell-Aleff PC, Meyer FB. Rhabdoid meningioma: An aggressive variant. Am J Surg Pathol. 1998. 22: 1482-90
38. Plotz PH, Klippel JH, Decker JL, Grauman D, Wolff B, Brown B. Bladder complications in patients receiving cyclophosphamide for systemic lupus erythematosus or rheumatoid arthritis. Ann Intern Med. 1979. 91: 221-3
39. Pompili A, Derome PJ, Visot A, Guiot G. Hyperostosing meningiomas of the sphenoid ridge-clinical features, surgical therapy, and long-term observations: Review of 49 cases. Surg Neurol. 1982. 17: 411-6
40. Pons-Estel GJ, Ugarte-Gil MF, Alarcón GS. Epidemiology of systemic lupus erythematosus. Expert Rev Clin Immunol. 2017. 13: 799-814
41. Radis CD, Kahl LE, Baker GL, Wasko MC, Cash JM, Gallatin A. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum. 1995. 38: 1120-7
42. Richardson TT, Cohen PR. Subacute cutaneous lupus erythematosus: Report of a patient who subsequently developed a meningioma and whose skin lesions were treated with isotretinoin. Cutis. 2000. 66: 183-8
43. Ringel F, Cedzich C, Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007. 60: 214-21
44. Roser F, Nakamura M, Bellinzona M, Rosahl S, Ostertag H, Samii M. The prognostic value of progesterone receptor status in meningiomas. J Clin Pathol. 2004. 57: 1033-7
45. Ruiz-Irastorza G, Ugarte A, Egurbide M, Garmendia M, Pijoan J, Martinez-Berriotxoa A. Antimalarials may influence the risk of malignancy in systemic lupus erythematosus. Ann Rheum Dis. 2007. 66: 815-7
46. Sade B, Chahlavi A, Krishnaney A, Nagel S, Choi E, Lee JH. World Health Organization Grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery. 2007. 61: 1194-8
47. Saeed P, van Furth WR, Tanck M, Kooremans F, Freling N, Streekstra GI. Natural history of spheno-orbital meningiomas. Acta Neurochir (Wien). 2011. 153: 395-402
48. Sandalcioglu IE, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: Interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005. 33: 260-6
49. Sankaran S, Sankaralingam R. Meningioma and Lupus-A Deadly Duo!-A Report of 2 Cases. 2015. 3: 1-4
50. Schwartzbaum J, Jonsson F, Ahlbom A, Preston-Martin S, Lönn S, Söderberg KC. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. Int J Cancer. 2003. 106: 423-8
51. Sekhar LN, Møller AR. Operative management of tumors involving the cavernous sinus. J Neurosurg. 1986. 64: 879-89
52. Shrivastava RK, Sen C, Costantino PD, Rocca RD. Sphenoorbital meningiomas: Surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005. 103: 491-7
53. Silman AJ, Petrie J, Hazleman B, Evans S. Lymphoproliferative cancer and other malignancy in patients with rheumatoid arthritis treated with azathioprine: A 20 year follow up study. Ann Rheum Dis. 1988. 47: 988-92
54. Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: A systematic review and meta-analysis. Arthritis Res Ther. 2018. 20: 270
55. Tallbacka K, Pettersson T, Pukkala E. Increased incidence of cancer in systemic lupus erythematosus: A Finnish cohort study with more than 25 years of follow-up. Scand J Rheumatol. 2018. 47: 461-4
56. Terrier LM, Bernard F, Fournier HD, Morandi X, Velut S, Hénaux PL. Spheno-orbital meningiomas surgery: Multicenter management study for complex extensive tumors. World Neurosurg. 2018. 112: e145-56
57. Wang XQ, Mei GH, Zhao L, Li ST, Gong Y, Zhong J. Clinical features and treatment of intracranial chordoid meningioma: A report of 30 cases. Histopathology. 2013. 62: 1002-17
58. Weckerle CE, Niewold TB. The unexplained female predominance of systemic lupus erythematosus: Clues from genetic and cytokine studies. Clin Rev Allergy Immunol. 2011. 40: 42-9
59. Yoo BW, Ahn SS, Pyo JY, Byun SJ, Song JJ, Park YB. Brain meningioma in a patient with systemic lupus erythematosus. Yeungnam Univ J Med. 2016. 33: 159-61
60. Zhou Y, Xie Q, Gong Y, Mao Y, Zhong P, Che X. Clinicopathological analysis of rhabdoid meningiomas: Report of 12 cases and a systematic review of the literature. World Neurosurg. 2013. 79: 724-32