- Department of Neurosurgery, The Center for Advanced Neurology and Neurosurgery (CEANNE Brazil), Brazil,
- Department of Anatomy, UFRGS, Rio Grande do Sul, Brazil,
- Department of Neurosurgery, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Sao Paulo, Brazil.
Correspondence Address:
Eberval Gadelha Figueiredo
Department of Neurosurgery, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Sao Paulo, Brazil.
DOI:10.25259/SNI_708_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Gustavo Rassier Isolan1, Marco Antonio Stefani2, Felipe Luis Schneider2, Humberto Alves Claudino2, Yang Han Yu3, Gil Goulart Choi3, Joao Paulo Mota Telles3, Nícollas Nunes Rabelo3, Eberval Gadelha Figueiredo3. Hippocampal vascularization: Proposal for a new classification. 06-Nov-2020;11:378
How to cite this URL: Gustavo Rassier Isolan1, Marco Antonio Stefani2, Felipe Luis Schneider2, Humberto Alves Claudino2, Yang Han Yu3, Gil Goulart Choi3, Joao Paulo Mota Telles3, Nícollas Nunes Rabelo3, Eberval Gadelha Figueiredo3. Hippocampal vascularization: Proposal for a new classification. 06-Nov-2020;11:378. Available from: https://surgicalneurologyint.com/surgicalint-articles/10371/
Abstract
Background: Anatomy of the hippocampal arterial supply is key to successful surgeries in this area. The goal of the current study is to present the results we obtained from our microsurgical dissections of the temporal lobe and to propose a new classification for the hippocampal arteries (HAs).
Methods: Fifty-six brain hemispheres were analyzed. All dissections in this study were made using 3–40× at the surgical microscope.
Results: The hippocampal arterial vasculature can be divided into six groups, according to their frequencies: Group A: mixed arterial vasculature originating from the anterior choroidal artery anterior choroidal artery, posterior cerebral artery (PCA), anterior infratemporal artery (AIA), and splenic artery (SA). Group B: Main origin at the temporal branches – main inferotemporal trunk, middle inferotemporal artery, posterior inferotemporal artery, AIA, or main branch of PCA. Group C: AIA as the main branch of the hippocampus. Group D: HAs originating from the main branch of PCA. Group E: A single hippocampal artery with the origin at the main branch of PCA. This single artery covered all of the structure and is named Ushimura’s artery. Group F: The hippocampal vessels arose exclusively from the parieto-occipital artery, calcarine artery (CA), and the SA.
Conclusion: This study proposes a new classification for the hippocampal vascularization, according to the origin of HAs. One of the groups has not yet been described in the literature – in which the HAs arise from the parieto-occipital artery, SA, and CA.
Keywords: Classification, Hippocampus, Neuroanatomy
INTRODUCTION
The temporal lobe is the most heterogeneous lobe of the human brain. It is formed by the allocortex and a combination of structures from the limbic system. The delimitation between the temporal isocortex and part of the limbic system is formed by the mesocortex.[
(HAs).
MATERIALS AND METHODS
Twenty-seven cadaveric heads were analyzed. Each head was placed in a Sugita head holder, extended, and rotated to simulate the surgical position. The pterional-transsylvian approach to the mesiotemporal region was performed according to the previous description by Yasargil et al. (2010).[
Forty brain hemispheres were filled with Batson fluid for 20 min for polymerization reaction. Fourteen hemispheres were filled with commercial latex plus PVA pigment. Other two hemispheres were filled with pigmented gelatin and used to examine the pattern of smaller caliber vessels. To preserve the brain’s anatomical characteristics, the postmortem period for removal and repletion was no longer than 18 h.
For adequate perfusion of the pigmented media, a cannula with adequate caliber was used, after previous perfusion with abundant saline solution (0.9%). All perfusions were pressure controlled. This procedure avoids vessel ruptures and perfusion media extravasations.[
After vascular injection, the hemispheres were fixated in a solution containing formaldehyde (25% [v/v]). Surgical microscopes were used in all phases of the study for more precise manipulation and more effective preservation of the anatomical parts. Statistical analyses were performed with Student’s t-test and analysis of variance. IRB approval was obtained for this study.
RESULTS
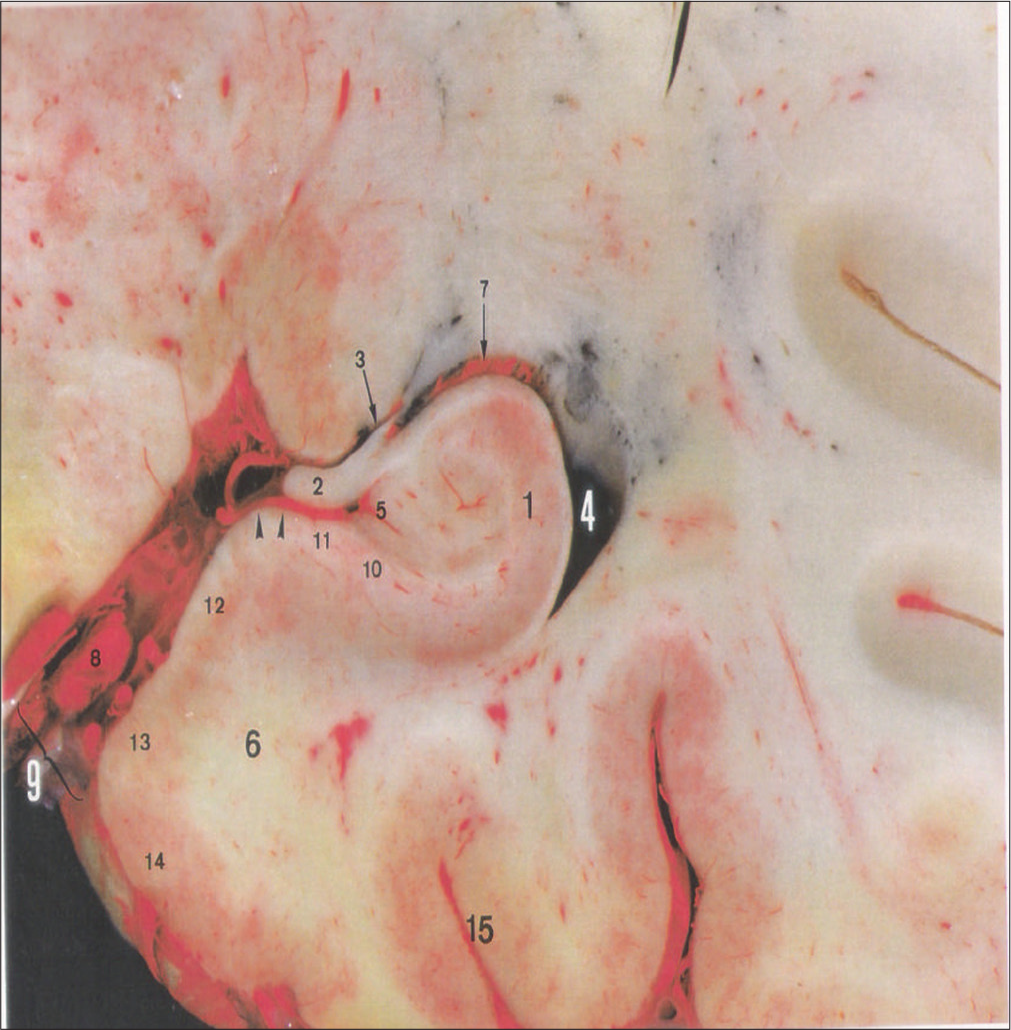
An anterior view of a coronal slice at the level of the hippocampus body is shown in [
Figure 1:
An anterior coronal slice at the level of the hippocampus body shows a general overview. This curved structure, which has been compared to a horn or a seahorse, is a common target in tumor and epilepsy surgery. The hippocampus is found in the dorsal aspect of the parahippocampal gyrus, consisting of the floor of the inferior horn of the lateral ventricle. Most of the hippocampus body is covered by the choroidal plexus. The fimbria represents the medial limit of the floor of the inferior horn. Outside of the ventricles, a vascular mesh covers the subiculum, where hippocampal arteries have significant anatomical variations. This slice has been perfused with latex and pigments. 1: hippocampus, 2: fimbriae, 3: choroidal fissure, 4: inferior horn of the lateral ventricle, 5: dentate gyrus, 6: parahippocampal gyrus, 7: choroidal plexus, 8: posterior cerebral artery, 9: ambiens cistern, 10: prosibiculum , 11: subiculum, 12: presubiculum, 13: parasubiculum, 14: entorhinal cortex, 15: collateral sulcus.
In the 27 left hemispheres, 124 HAs were found (mean = 4.59; mode = 4; range = 3–7 per hemisphere). In the 27 right hemispheres, 126 HAs were found (mean = 4.67: mode = 5; range = 2–10 per hemisphere). The mean difference of HAs observed between the left and right hemispheres was 1.48 arteries, with a minimum of 0 and a maximum of 7 HAs.
Of the 250 HAs identified in this study, 81 (32.4%) arose from trunks, not directly from main arteries such as the posterior cerebral artery (PCA), parieto-occipital artery (POA), or calcarine artery (CA). These trunks were the uncohippocampal trunk supplying the uncus and the head of the hippocampus; and the parahippocampal-hippocampal trunk, supplying the parahippocampal gyrus, body, and head of the hippocampus.
In the 54 hemispheres, we found 36 trunks. There were 18 uncohippocampal trunks – 3 trunks (16.7%) arising from the anterior choroidal artery (AChA), 7 trunks (38.9%) from the P2 segment of the PCA, 1 trunk (5.5%) from the parieto-occipital artery, and 7 trunks (38.9%) arising from the anterior inferior temporal artery. There were 18 parahippocampalhippocampal trunks – 1 trunk (5.56%) arising from the common inferior temporal artery, 11 trunks (61.11%) from the anterior inferior temporal artery (AITA), and 6 trunks (33.33%) from the posterior inferior temporal artery (PITA).
The arteries that supply the hippocampus are located in the extraventricular portion and cannot be seen in the ventricular cavity. On the dorsal side of the parahippocampal gyrus (subiculum), the HAs assume a parallel position to the hippocampus, where they give rise to straight vessels that run perpendicularly toward the hippocampus. These straight vessels, regardless of their origins, penetrate the hippocampus at the level of the dentate gyrus. They run through the ambient cistern and the transverse fissure in most cases, ending up in the hippocampus through the hippocampal and fimbriodentate sulci. These vessels have a straight path and were observed in 43 hemispheres (79.63%): twenty-two in the left hemispheres (40.74%) and 21 in the right hemispheres (38.89%). This description could only be confirmed after the removal of the fimbriae. Most of these straight vessels are collateral ramifications of the HA (13, 16). These vessels penetrate, in part, through the denticulate margin, in the area between the hippocampal sulcus and the fimbriodentate sulcus. Some of these vessels penetrate the subiculum, and others run around the fimbriae and the hippocampus.
Proposed classification for hippocampal vascularization
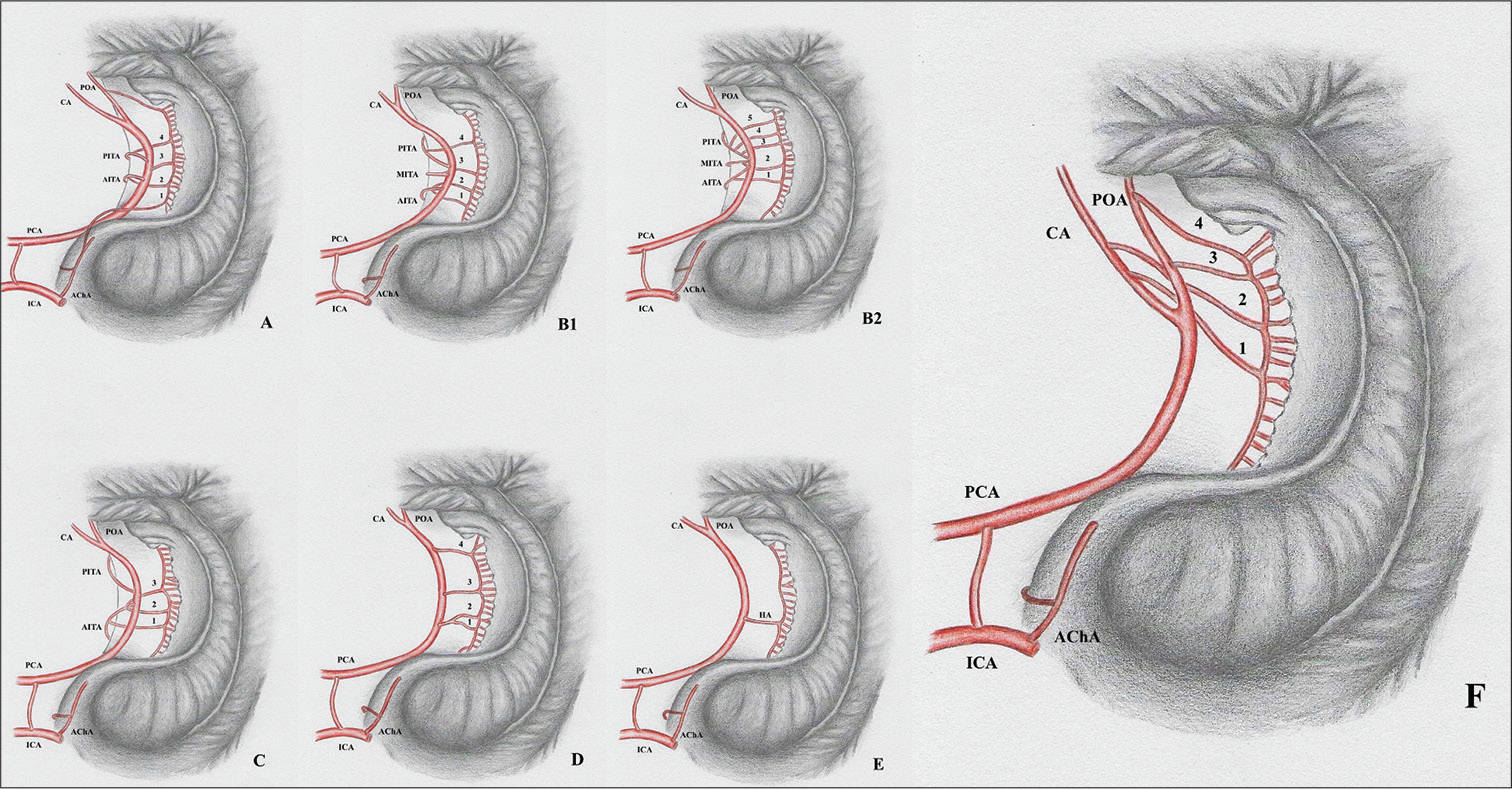
The hippocampal arterial vasculature may be divided into six groups, according to their frequencies [
Figure 2:
Intra and extraventricular view of the hippocampus vascularization groups. Hippocampal arteries present significant anatomical variations that have direct implications to surgical procedures such as amygdalohippocampectomy. According to their origin, the hippocampal vascularization patterns can be categorized in six different groups. A: Group A presents mixed irrigation, with the hippocampal arteries originating from the anterior choroidal, posterior cerebral, anterior inferior temporal, and splenial arteries. B1: Group B presents hippocampal arteries originating from the inferior temporal branches and PCAs main trunk. B2: Also Group B, but in a situation where the inferior temporal arteries arise from a common trunk. Arrowhead identifying a common inferior temporal artery. C: Group C presents the anterior inferior temporal artery as the main feeder of the hippocampus. D: Group D hippocampal arteries originate from the posterior cerebral arteries’ main trunk. The hippocampal arteries are the largest contributors to the hippocampus blood supply in this group. E: The trunk of the posterior cerebral artery originates Uchimura’s artery – a vessel that irrigates practically the whole hippocampus. F: A new anatomical variation, in which the hippocampal vessels arise exclusively from the POA, CA, and the SA. CA: Calcarine artery; ICA: Internal carotid artery, PCA: Posterior cerebral artery, POA: Parieto-occipital artery, AITA: Anterior inferior temporal artery, MITA: Medial inferior temporal artery, PITA: Posterior inferior temporal artery, AChA: Anterior choroidal artery. 1, 2, 3, and 4: Hippocampal arteries.
Group A: Mixed arterial vasculature originating from the AChA, PCA, anterior infratemporal artery (AIA), and splenic artery (SA). Group B: Main origin at the temporal branches – main inferotemporal trunk (MIT), middle inferotemporal artery (MIA, posterior inferotemporal artery (PIA), AIA, or main branch of PCA. Group C: AIA as the main branch of the hippocampus. Group D: HAs originating from the main branch of PCA. Group E: A single hippocampal artery (HA) with the origin at the main branch of PCA. This single artery covered all of the structure and is named Ushimura’s artery. Group F: The hippocampal vessels arose exclusively from the POA, CA, and the SA.
Group A was present in 25 hippocampi (46.3%). Group B of vasculature was present in 11 (20.4%), Group C in 8 hippocampi (14.8%), Group D in 7 (13%), and Group E in 2 hemispheres (3.7%). Group F was observed in one left hemisphere (1.8%), and this anatomical variation has not been described yet. The frequencies of different types of arterial vascularization did not differ between the left or right hippocampi in this study.
AChA
In all of the analyzed hemispheres, AChA had its origin in the posterior wall of the internal carotid artery (ICA).
We observed only one unusual case, in which the AChA ran through the transverse fissure dorsally to the parahippocampal gyrus (presubiculum) and reached the cortical area posteriorly to the splenium of the corpus callosum. Along its entire course, this AChA gave rise to only one branch of the HA. This HA supplied the body and tail of the hippocampus.
The AChA was identified in all the hemispheres analyzed in our study and gave branches to the hippocampus in 17 of 54 cases (31.5%). Even though approximately one-third of the cases had a HA coming from the AChA, in 28 cases (51.8%), the material injected through the AChA was identified in the vascular territory of the hippocampus. The HAs from the AChA have a similar conformation to the HAs from the PCA and follow the hippocampal sulcus in the uncus region as well. Our findings did not show any variation in the diameter or length of the HAs, regardless of their origins. Even so, the arteries from the AChA supplied mainly the head of the hippocampus.
PCA
The PCA branches are divided into three groups: central (I), ventricular and choroid plexus (II), and cerebral branches (III) (31, 32). We focused on the second and third groups since they are related to hippocampal vascularization. The posterolateral and posteromedial choroid arteries are among these branches. They supply the superior tela choroidea, choroid plexus of the lateral and third ventricle, thalamus, and the internal region of the caudate nucleus.
This group includes the HAs, AITA, MITA, PITA, POA, CA, and SA. They supply the external occipital and ventral faces of the hemispheres, comprehending the inferotemporal, lateral occipitotemporal, medial occipitotemporal, and parahippocampal gyri. On the medial face, they supply the uncus, hippocampus, superior surface of the parahippocampal gyrus, and the posterior areas of parietal and occipital lobes, including the precuneus and cuneus.
HA and its parent vessels
The HA is one of PCAs first branches after P1 and was present in all hemispheres. In 26 hemispheres (48.15%), the HA arose directly from the main trunk of the PCA. This trunk gave rise to 44 HAs (17.6%), with 19 HAs (7.6%) in the left hemispheres, and 25 HAs (10%) in the right hemispheres.
The MIT was observed in 12 (22.2%) of all the hemispheres analyzed. In 2 hemispheres (3.7%), the MIT originated 2 HAs (0.8%). The anterior inferotemporal artery (AIA) was present in 50 hemispheres (92.6%). In 31 cases (57.4%), it branched arteries toward the hippocampus, with a total number of 49 HAs (19.6%). The MIA was present in 9 hemispheres (16.7%) and branched toward the hippocampus in only 1 case (0.4%). The PIA was present in 46 hemispheres (85.2%) and gave rise to 31 HAs (12.4%) in 17 cases (31.5%).
The POA was found in 52 hemispheres (96.3%), originating from the PCA, at the level of P2 or P3 segments. This artery sent 43 HAs (17.2%) to the hippocampus in 26 (48.2%) of the hemispheres analyzed. The CA is another branch of the PCA, located medially and ventrally to the POA. It was identified in 52 hemispheres (96.3%) and, in 17 hemispheres (31.5%), it sent 30 arteries (12.0%) to the hippocampus. The SA was identified in 35 hemispheres (64.8%). In 29 hemispheres (53.7%), it sent 33 HAs (13.2%) to the hippocampus. SA originated from the P3 segment in all cases, except for one, in which it descended from P2. To the best of our knowledge, this variation has not yet been described in the literature.
Vascular arcades and anastomoses
Anastomoses connecting the AChA or its branches to the PCA, POA, and CA (or their branches) were observed in 23 hemispheres (42.6%). These anastomoses connected the uncal branches of the AChA to the HAs of the cited vessels. Anastomoses connecting two HAs - one coming from the AChA and the other from the PCA, POA, or CA – were found in 5 hemispheres (9.3%). In most cases, the anastomoses appeared with an arcuate conformation.
At the level of the choroid plexus of the lateral ventricle’s inferior horn, anastomoses were connecting the AChA or its branches to posterior choroidal arteries descending from the PCA in 26 hemispheres (48.2%). Both lateral posterior choroidal branches and distal AChA branches provide blood supply to important structures, such as the lateral geniculate nucleus, and therefore, it is essential to recognize these anastomoses to avoid visual field deficits.
DISCUSSION
The hippocampus has been one of the most widely studied structures of the brain. Siege for spatiotemporal and declarative memory, its study led to the understanding of processes as diverse as neuronal networks and long-term potentiation. It is also of major interest in epilepsy, from both clinical and surgical perspectives, as well as tumor surgery.[
From a neurosurgical point of view, knowledge of the hippocampus and its arterial irrigation is crucial to perform successful surgeries while maintaining intact the important structures surrounding the operative field.[
We found a mean of 4.63 HAs per hippocampus, similar to previous works.[
In the 54 hemispheres studied, we found 36 trunks – 18 uncohippocampal and 18 parahippocampal-hippocampal. In a study with 30 brain hemispheres, Erdem et al.[
The MIT was observed in 12 (22.2%) of all the hemispheres analyzed. In 2 hemispheres (3.7%), it originated 2 HAs (0.8%). The previous studies have shown frequencies between 10 and 16% for its occurrence and 2.1% for it being the HAs origin.[
The AIA was present in 50 hemispheres (92.6%). In 31 cases (57.4%), it branched arteries toward the hippocampus, with a total number of 49 HAs (19.6%). The previous studies identified AIAs in 63.3–84% of the hemispheres. In 64% of the cases,[
The PIA was present in 46 hemispheres (85.2%) and gave rise to 31 HAs (12.4%) in 17 cases (31.5%). The previous studies have identified the PIA in 96% of the cases (31), with it contributing to the hippocampal vascularization in 43.3% of the cases (13). The POA was found in 52 hemispheres (96.3%), originating from the PCA, at the level of P2 or P3 segments. This artery sent 43 HAs (17.2%) to the hippocampus in 26 (48.2%) of the hemispheres analyzed. The previous studies have described the POA with a lower contribution to the hippocampal vascularization – only 26.6% of the POAs sent branches to the hippocampus.[
The CA is another branch of the PCA, located medially and ventrally to the POA. It was identified in 52 hemispheres (96.3%) and, in 17 hemispheres (31.5%), it sent 30 arteries (12.0%) to the hippocampus. This terminal branch of the PCA has been described with a frequency of 100%, with lower (3.3% of the cases) contribution to the hippocampal vasculature. The SA was identified in 35 hemispheres (64.8%). In 29 hemispheres (53.7%), it sent 33 HAs (13.2%) to the hippocampus. The previous studies have described the SA occurring with a frequency of 62.0%, contributing to the hippocampal vascularization in 63.3% of the cases, with hippocampal vessels descending from a curvature at the ventricular portion of the hippocampus in 36.6% of the cases.[
The HA was present in all the hemispheres analyzed, as well as in all previous studies searched. In 26 hemispheres (48.15%), the HA arose directly from the main trunk of the PCA. In other studies, the PCAs originated HAs with frequencies between 56.6 and 64% of the cases.[
The AChA was identified in all the hemispheres analyzed in our study and gave branches toward the hippocampus in 17 of 54 cases (31.5%). The AChA has been previously described as the origin of, at least, one HA in 8.8–17.1% of the cases.[
We also described anastomoses connecting the AChA or its branches to the PCA, POA, and CA in 42.6% of patients, showing an arcuate conformation in most cases. The previous studies have observed anastomoses connecting two HAs in 22.8% of the cases.[
Proposed classification
Erdem et al. (1993)[
In the third group, the HAs arose from the AITA and from the main trunk of the PCA. It was present in 3 cases (10%). In this study, we observed a similar group – the Group C – which had the AIA as the main branch of the hippocampus. In the fourth group, the main vessel that supplied the hippocampus was a single ramification that arose from the main branch of the PCA (Uchimura’s artery). In our study, this type of vasculature was observed in 2 hemispheres (3.7%), corresponding to Group E.
The fifth group, in which the AChA was the most important vessel in the hippocampal vascularization, was not observed in our study. It is worth mentioning that, in our study, we found an additional pattern of vascularization (Group F), in which the hippocampal vessels arose from the POA, SA, and CA. These results have not yet been described in the literature thus far. The new proposed classification is, therefore, broader than the previous one, encompassing a new group not yet identified.
CONCLUSION
Anatomy of the hippocampal arterial supply is key to successful surgeries in this area. This study proposes a new classification for the hippocampal vascularization, according to the origin of HAs. Indeed, this paper identified one pattern that has not yet been in the literature thus far: HAs that arise from the parieto-occipital artery, SA, and CA. At the best of our knowledge, this study encompasses the largest series of hippocampal vascularization in the literature.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Erdem A, Yaşargil MG, Roth P. Microsurgical anatomy of the hippocampal arteries. J Neurosurg. 1993. 79: 256-65
2. Goetzen B, Sztamska E. Comparative anatomy of the arterial vascularization of the hippocampus in man and in experimental animals (cat, rabbit and sheep). Neuropatol Pol. 1992. 30: 173-84
3. Hanstede JG, Gerrits PO. A new plastic for morphometric investigation of blood vessels, especially in large organs such as the human liver. Anat Rec. 1982. 203: 307-15
4. Knierim JJ. The hippocampus. Curr Biol. 2015. 25: R1116-21
5. Laine E. Arterial Vertebro-Basilar Aneurysms. Prog Brain Res. 1968. 30: 323-46
6. Marinković S, Milisavljević M, Puškaš L. Microvascular anatomy of the hippocampal formation. Surg Neurol. 1992. 37: 339-49
7. Milisavljević M, Marinković S, Lolić-Draganić V, Djordjević L. Anastomoses in the territory of the posterior cerebral arteries. Acta Anat (Basel). 1986. 127: 221-5
8. Muller J. Arterial vascularization of the human hippocampus 1. Extracerebral relationships. Arch Neurol. 1965. 13: 45-7
9. Nakai Y, Masutani H, Moriguchi M, Matsunaga K, Kato A, Maeda H. Microvasculature of normal and hydropic labyrinth. Scanning Microsc. 1992. 6: 1097-103
10. Tatu L, Vuillier F. Structure and vascularization of the human hippocampus. Front Neurol Neurosci. 2014. 34: 18-25
11. van der Zwan A, Hillen B. Araldite F as injection material for quantitative morphology of cerebral vascularization. Anat Rec. 1990. 228: 230-6
12. Wen HT, Rhoton AL, de Oliveira E, Cardoso AC, Tedeschi H, Baccanelli M. Microsurgical anatomy of the temporal lobe: Part 1: Mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery. 1999. 45: 549-92
13. Wieser HG, Yaşargil MG. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol. 1982. 17: 445-57
14. Wilson CL, Isokawa M, Babb TL, Crandall PH, Levesque MF, Engel J. Functional connections in the human temporal lobe. II, Evidence for a loss of functional linkage between contralateral limbic structures. Exp Brain Res. 1991. 85: 174-87
15. Witter MP, Kleven H, Flatmoen AK. Comparative contemplations on the hippocampus. Brain Behav Evol. 2017. 90: 15-24
16. Yaşargil MG, Krayenbühl N, Roth P, Hsu SP, Yaşargil DC. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010. 112: 168-85
17. Yaşargil MG, Türe U, Yaşargil DC. Impact of temporal lobe surgery. J Neurosurg. 2004. 101: 725-38
18. Zeal AA, Rhoton AL. Microsurgical anatomy of the posterior cerebral artery. J Neurosurg. 1978. 48: 534-59