- Neuroradiology Unit, Department of Biomedical, Dental Science and Morphological and Functional Images, Italy.
- Radiology Unit, Department of Biomedical, Dental Science and Morphological and Functional Images, Italy.
- Section of Neurosurgery, Department of Biomedical Sciences and Morphological and Functional Imaging, University of Messina, Messina, Italy.
- Section of Neurosurgery, Department of Biomedicine, Neurosciences and Advanced Diagnostic, University of Palermo, Palermo, Italy.
DOI:10.25259/SNI_151_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Karol Galletta, Michele Gaeta, Concetta Alafaci, Sergio Vinci, Marcello Longo, Giovanni Grasso, Francesca Granata. Hirayama disease – Early MRI diagnosis of subacute medullary ischemia: A case report. 16-May-2020;11:115
How to cite this URL: Karol Galletta, Michele Gaeta, Concetta Alafaci, Sergio Vinci, Marcello Longo, Giovanni Grasso, Francesca Granata. Hirayama disease – Early MRI diagnosis of subacute medullary ischemia: A case report. 16-May-2020;11:115. Available from: https://surgicalneurologyint.com/surgicalint-articles/10031/
Abstract
Background: Hirayama disease (HD) is a rare, benign, and self-limiting motor neuron disorder that results in selective motor impairment of the C7-T1 myotomes. It is characterized by progressive, unilateral, or bilateral asymmetric muscle atrophy of the distal upper extremities and myelopathy.
Case Description: A 23-year-old male presented with bilateral atrophy of the thenar/hypothenar eminences/ interosseous muscles, plus left-hand weakness. The cervical MRI documented subacute ischemic damage of the distal cervical cord. To rule out a tumor and reduce questionable cord compression, the patient underwent a C5–C6 anterior cervical discectomy and fusion (ACDF) immediately followed by a laminectomy with durotomy and to obtain a spinal cord biopsy. When the histology confirmed focal cord ischemia consistent with HD, it was clear that both operations were unnecessary.
Conclusion: Establishing the diagnosis of HD is based on clinical findings and MRI/flexion MR features which include the demonstration of an increased T2-weighted intramedullary cord signal, enlargement of the posterior epidural space, and segmental spinal cord atrophy. The presence of HD should be recognized as a “nonsurgical entity,” and conservative nonsurgical management should be employed.
Keywords: Amyotrophy, Hirayama disease, Magnetic resonance imaging
INTRODUCTION
Hirayama disease (HD) is a rare, benign, and self-limiting motor neuron disorder that typically involves selective motor impairment in the C7-T1 myotomes (e.g., involving the hands and forearms and related to cervical flexion maneuvers). It is attributed to chronic spinal cord damage occurring during repeated cervical flexion maneuvers and is characterized by progressive subacute unilateral or bilateral distal upper extremity myelopathy (i.e. painless amyotrophy).[
CASE REPORT
Clinical presentation
A 23-year-old Italian male presented with a 2-year history of progressive left greater than the right- hand weakness/ myelopathy. He exhibited left greater than the right-sided atrophy of the thenar/hypothenar eminences, and interosseous muscles. Motor nerve conduction/electromyographic studies showed left greater than the right-sided C7-T1 denervation, while sensory conduction studies were normal.
MRI findings
The patient underwent both neutral and flexion. A 1.5 Tesla cervical magnetic resonance imaging (MRI) showing diffuse lower cervical spinal cord swelling (C4–C7) most severe at C5–C6 with mild spondylosis and a high intrinsic T2-weighted cord signal (e.g. with punctate foci of restricted diffusion) [
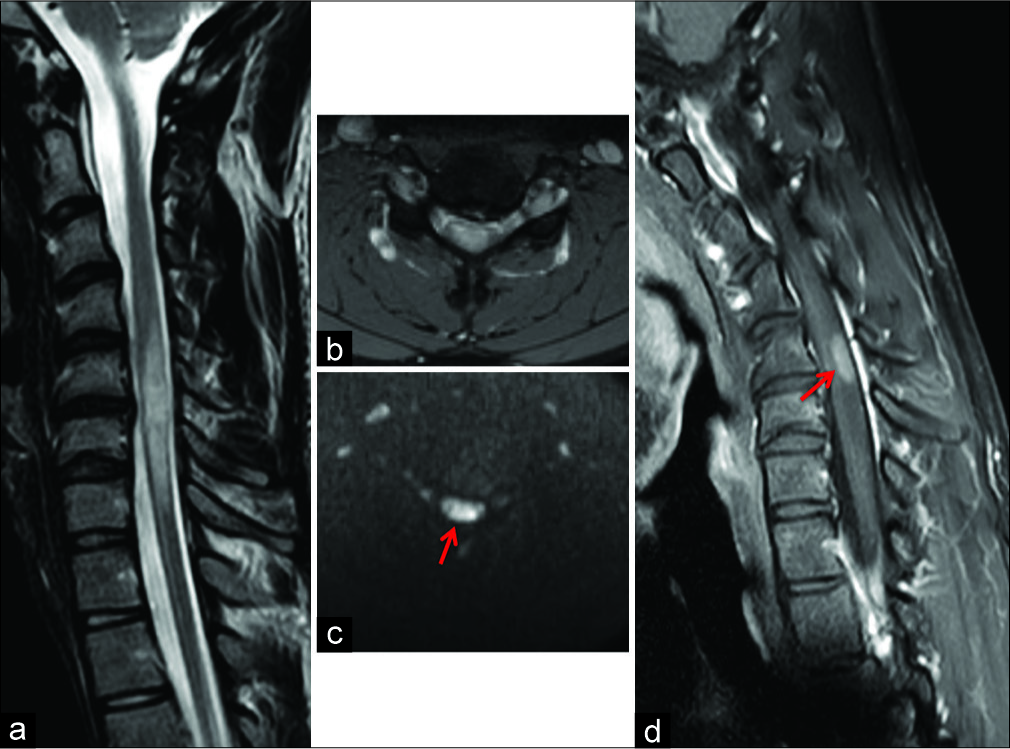
Figure 1:
(a) Sagittal T2-weighted FSE magnetic resonance imaging of the cervical spine in neutral position. Segmental intramedullary swelling with high signal intensity at C5 to C7 level and loss of cervical lordosis. (b,c) Neutral position axial T2-weighted GRE and axial DWI sequence at C5–C6 disc level showing segmental spinal cord hyperintensity with some foci of restricted diffusion (arrow). A median-paramedian disc herniation at the same level was also evident. (d) Sagittal contrast-enhanced T1-weighted FSE acquisition on neck flexion showing a partial enhancement of the spinal cord lesion at C5–C6 level (arrow).
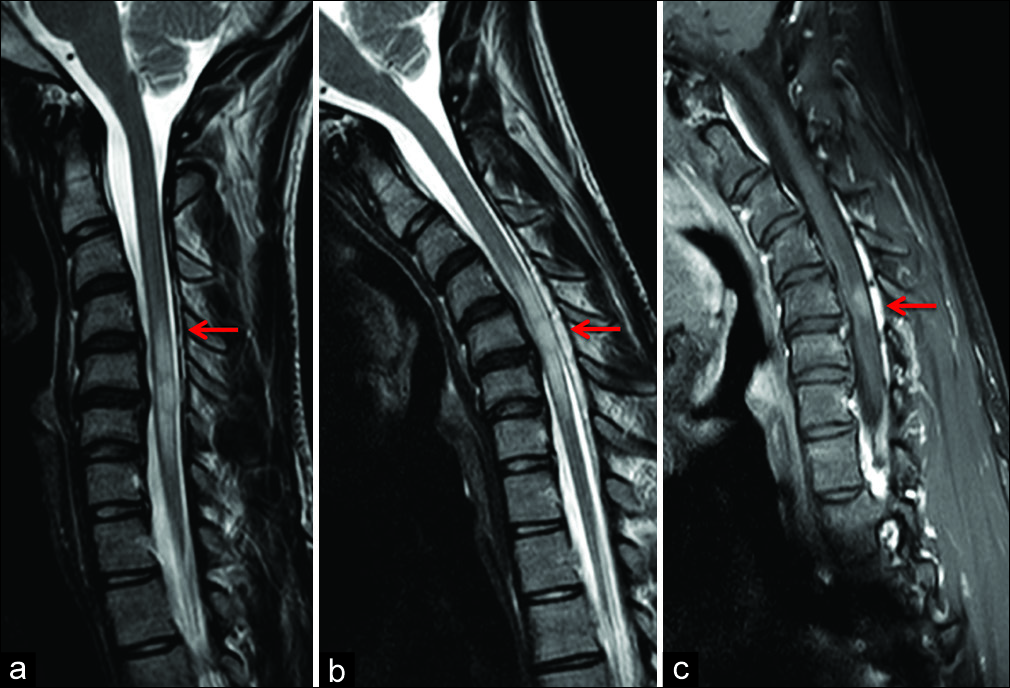
Figure 2:
(a) Sagittal T2-weighted FSE image in neutral position with evidence of posterior detachment of dural sac (arrow). (b) Flexion sagittal T2-weighted FSE images confirming anterior displacement of the posterior dura from C4 to C7 levels with spinal cord flattening and prominence of the posterior epidural space (arrow). (c) Sagittal contrast-enhanced T1-weighted FSE in flexion position with evidence of enhancement of the enlarged posterior epidural space (arrow).
The contrast the MR showed enhancement within the cord at C5–C6 level [
Surgery
The patient first underwent a C5–C6 ACDF to achieve anterior decompression. To exclude an intrinsic cord tumor, a cervical laminectomy with durotomy allowed for an intramedullary cord biopsy. The latter documented focal cord ischemia consistent with intrinsic HD.
Follow-up
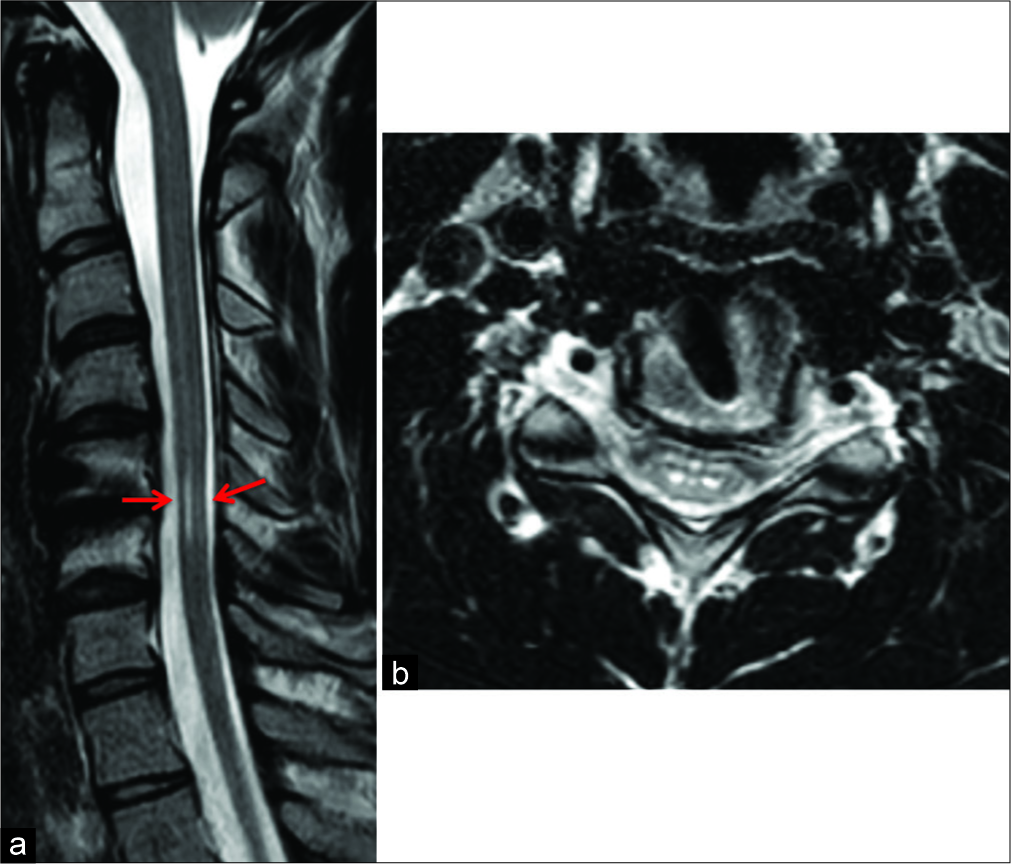
One year later, the cervical MRI demonstrated a reduction of the ischemic C5–C6 cord focus, and the patient clinically remained unchanged. Over the next 2 years, the subsequent MRI scans showed a mild progressive focal atrophy of the cord at the C5–C6 level with selective involvement of the anterior horns [
DISCUSSION
Etiology
HD, also called “Juvenile muscular atrophy of the distal upper extremities,” is a rare myelopathy involving the lower cervical spine characterized by an insidious onset of atrophy involving the C7, C8, and T1 myotomes, with sensory sparing.[
HD may be attributed to: (1) a disproportionate growth between the spinal column and cord or (2) repeated neck flexion with microcirculatory changes involving the anterior spinal artery result in ischemia.[
MRI findings
Dynamic cervical MRI scans play a critical role in the diagnosis of HD. The neutral MRI usually shows (1) localized lower cervical cord atrophy/asymmetric cord flattening, (2) a high intrinsic cord signal on T2-weighted images, and (3) an abnormal cervical curvature with loss of attachment between the posterior dural sac and subjacent lamina.[
Etiology of ischemia
The etiology of spinal cord ischemia is potentially related to passive dilation of the posterior epidural venous plexus, contributing to chronic ischemic damage of the anterior horns [
Surgery
There is no role for surgery in HD[
CONCLUSION
HD should be suspected in young males presenting with a slowly progressive myelopathy. Based on the MRI findings, the diagnosis of HD, a neurodegenerative disorder (i.e., intrinsic cord changes without significant extrinsic compression), should be considered averting unnecessary surgery.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agundez M, Rouco I, Barcena J, Mateos B, Barredo J, Zarranz JJ. Hirayama disease: Is surgery an option?. Neurologia. 2015. 30: 502-9
2. Hirayama K. Juvenile muscular atrophy of distal upper extremity (Hirayama disease). Intern Med. 2000. 39: 283-90
3. Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology. 2000. 54: 1922-6
4. Hirayama K, Tomonaga M, Kitano K, Yamada T, Kojima S, Arai K. Focal cervical poliopathy causing juvenile muscular atrophy of distal upper extremity: A pathological study. J Neurol Neurosurg Psychiatry. 1987. 50: 285-90
5. Lapresle J. Clinical and neuropathological aspects of circulatory disorders of the spinal cord. Bull Schweiz Akad Med Wiss. 1969. 24: 512-28
6. Raval M, Kumari R, Dung AA, Guglani B, Gupta N, Gupta R. MRI findings in Hirayama disease. Indian J Radiol Imaging. 2010. 20: 245-9
7. Tokumaru Y, Hirayama K. Cervical collar therapy for juvenile muscular atrophy of distal upper extremity (Hirayama disease): Results from 38 cases. Rinsho Shinkeigaku. 2001. 41: 173-8