- Department of Neurosurgery, ISSSTE, Mexico City, Mexico,
- Department of Neurosurgery, Hospital Petrona V. de Cordero, San Fernando,
- Laboratory of Microsurgical Neuroanatomy, Second Chair of Gross Anatomy, School of Medicine, University of Buenos Aires, Buenos Aires,
- Department of Pathology of the Faculty of Medicine, Buenos Aires University, Buenos Aires, Argentina,
- Department of Neurosurgery, Sao Paulo Federal University, Sao Paulo, Brazil,
- Department of Neurosurgery, Hospital Padilla de Tucuman, San Miguel de Tucuman, Argentina,
- Neural Dynamics and Modulation Lab, Cleveland Clinic, Ohio, United States,
- Laboratory of Microsurgical Neuroanatomy, Second Chair of Gross Anatomy, University of Buenos Aires, Buenos Aires, Argentina.
Correspondence Address:
Gerardo Marín, Neural Dynamics and Modulation Lab, Cleveland Clinic, Ohio, United States.
DOI:10.25259/SNI_1022_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jonathan Samuel Zúñiga Córdova1, Mickaela Echavarría Demichelis2, Forlizzi Valeria3, Gustavo Garavaglia4, Feres Chaddad5, Carlos Castillo Rangel1, Jaime Ordóñez-Granja1, Alvaro Campero6, Gerardo Marín7, Matias Baldoncini8. Histological changes of vascular clipping in Wistar rats. 02-Dec-2022;13:561
How to cite this URL: Jonathan Samuel Zúñiga Córdova1, Mickaela Echavarría Demichelis2, Forlizzi Valeria3, Gustavo Garavaglia4, Feres Chaddad5, Carlos Castillo Rangel1, Jaime Ordóñez-Granja1, Alvaro Campero6, Gerardo Marín7, Matias Baldoncini8. Histological changes of vascular clipping in Wistar rats. 02-Dec-2022;13:561. Available from: https://surgicalneurologyint.com/surgicalint-articles/12039/
Abstract
Background: During aneurysm microsurgery, the aneurysmal sac is excluded from circulation by placing one or more clips at the base of the aneurysm. In some cases of complex aneurysms or subarachnoid hemorrhage history, transient clipping before definitive clipping is necessary. The closing force of the transient clip is less than the permanent clip; however, it is sufficient to stop circulation to the aneurysmal sac. The aim of the following work is to analyze and describe histological changes caused by transient and permanent clipping of the abdominal aorta in Wistar-type rats, to study the correlation between the closing force of the clip and the time, it remains on the vascular tissue structures.
Methods: Six groups were formed, with 10 rats each, whereby temporary clipping of the abdominal aorta was performed with subsequent sampling of the site where the vascular clip was placed. The groups were: control and temporary clipping with: 2, 5, 10, and 15 and permanent clipping with 5 min.
Results: Resection samples of the 3 μm thick aorta were obtained through the routine histological technique and special histochemical techniques (Masson’s Trichrome and orcein) from the six groups. Transmural changes were found from Group II–VI.
Conclusion: There is a vascular histological effect after both transient and permanent clipping. The sum of time and strength of the clip induce vascular changes visible at 5 min.
Keywords: Endothelial lesion, Histological changes, Vascular clipping, Wistar rats, Neuropathology
INTRODUCTION
Intracranial aneurysms are a vascular abnormality that causes focal dilation in the arterial wall, secondary to a loss of the internal elastic lamina. They are usually found in areas of arterial bifurcation of the anterior circulation of the Willis polygon. They have an incidence of 1–2% of the world population, being responsible for 80–85% of cases of nontraumatic subarachnoid hemorrhage (SAH). The peak age when presented corresponds to 40–60 years of age, with a slight preference for the female gender, 3:1.[
There are two types of treatments for brain aneurysms: endovascular or microsurgical treatment. The aim of microsurgery is to exclude the aneurysmal sac from circulation by placing one or more clips at the base of the aneurysm. For some cases of complex aneurysms or SAH history, transient clipping before definitive clipping is necessary. The closing force of the transient clip is less than the permanent clip; however, it is sufficient to stop circulation to the aneurysmal sac.[
The aim of the following work is to analyze and describe the histological changes caused by the transient and permanent clipping of the abdominal aorta in Wistar-type rats, to study the correlation between the closing force of the clip and the time, it remains on the structures of the vascular tissue.
MATERIALS AND METHODS
A descriptive, experimental, cross-sectional, and prospective study was carried out in a group of 60 WISTAR-type rats, weighing an average of 400 g.
In addition, a comprehensive bibliographic search was carried out on the PubMed platform using the following keywords: histological vascular change, temporal clipping, arterial wall, endothelial lesion, and vascular clip; obtaining only 12 articles for use from 1979–2022, as well as use of bibliography from the Science Direct platform for the management and anatomy of murine models in the laboratory.
The procedures were performed in the Laboratory of Microsurgical Neuroanatomy on the Second Anatomy Class in the School of Medicine at the University of Buenos Aires. The experimental work was approved by the Ethics Committee for the School of Pharmacy and Biochemistry at the Institute of Medical Sciences (Cudap number 35788/19, Resolution No. 2379). Furthermore, followed were the care standards described by the “International Guiding Principles for Biomedical Research Involving Animals” written by the Council for International Organizations of Medical Sciences (CIOMS) and the International Council for Laboratory Animal Science, Geneva 2012, was also met.
The work protocol, developed by our team, consisted of integrating six groups, with 10 rats each, on which temporary clipping of the abdominal aorta was performed with subsequent sampling of the site where the vascular clip was placed, which were arranged as follows:
Group I: Control group
Group II: Temporary clipping for 2 min
Group III: Temporary clipping for 5 min
Group IV: Temporary clipping for 10 min
Group V: Temporary clipping for 15 min
Group VI: Permanent clipping for 5 min
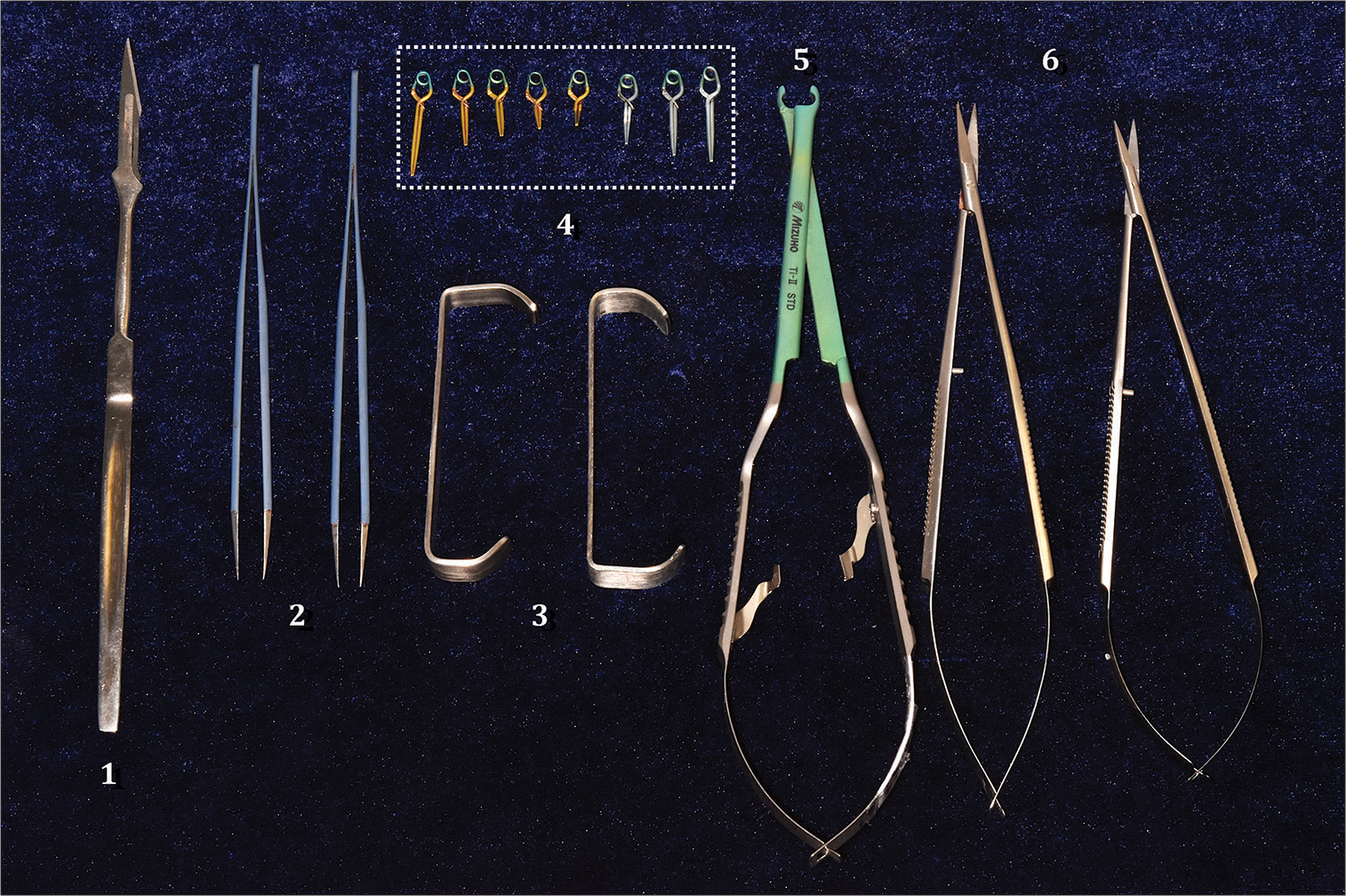
The surgical instruments used for this study were a NEWTON® brand microscope, MEC XXI model, 2000 series with 200 mm lens, macro and microsurgical instruments, such as scalpel handle and blade No. 11, microsurgical scissors, dissection scissors, straight tip dissection tweezers, needle holder, Farabeuf separators, five straight temporary clips of 0.69 N, 2 of 6.5 mm, 1 of 8 mm, 1 of 9.5 mm, and 1 of 12.5 mm, three permanent clips, two straight ones of 11.5 mm and 13.5 mm with pressure of 1.67 N and a curved one of 11 mm with 1.47 N, clip applicator tweezers, latex template, sterile gauze, 3-0 vicryl suture, buffered polypropylene, and formalin test tubes. The surgical procedure and anatomical description were recorded with a Nikon D5500 camera with a 62 mm lens and TRiOPO speedlight TR-15EX flash [
Figure 1:
Microsurgical instruments. (1) Scalpel Handle with Blade No. 11. (2) Watchmaker tweezers. (3) Farabeuf separator. (4) Sugita Type II permanent and temporary clips in dashed rectangle line: five straight temporary clips in gold color of 0.69 N, 2 of 6.5 mm, 1 of 8 mm, 1 of 9.5 mm, and 1 of 12.5 mm, three permanent clips in silver color, two straight ones of 11.5 mm and 13.5mm with pressure of 1.67 N, and one curved one of 11 mm with 1.47 N. (5) Clip applicator clamp. (6) Microsurgical scissors.
RESULTS
Anesthesia and positioning
The specimens were anesthetized with the following protocol: 80 mcg/kg of ketamine and 10 mg/kg of xylacin, intraperitoneally in the same syringe.
The rats were positioned in dorsal decubitus, with shaving of the abdominal region, asepsis and antisepsis with chlorhexidine, and marking of the incision. Once the procedure was completed, the specimens were euthanized with intraperitoneal barbiturate injection as required by international guidelines in the handling of laboratory animals.
Dissection of the abdominal region
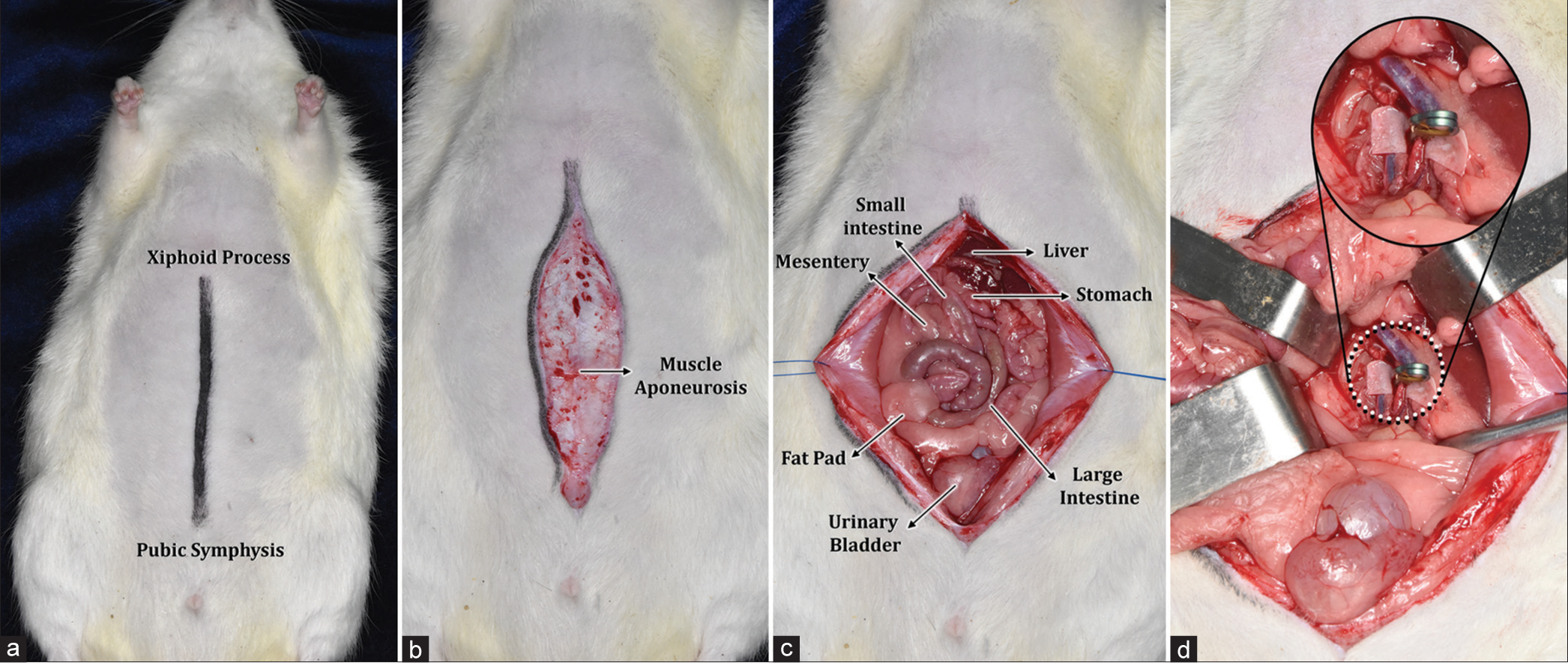
A linear incision was made from the xiphoid process as the upper limit to the pubic symphysis [
Procedure and methodology of temporary and permanent clippings
Dissection of the abdominal aorta was performed with the aforementioned technique and following the anatomical repairs mentioned. With adequate exposure thereof, a rectangular latex pad was placed below the blood vessel. In Group II, temporary clipping was initiated for 2 min with subsequent sampling of the vascular site where the clip was placed. The same procedure was repeated in subsequent specimens until the five working groups were completed, for 2, 5, 10, and 15 min of temporary clipping and 5 min of permanent clipping in Group VI. Finally, we worked with the control group, taking samples of the unharmed blood vessel. The sample was obtained by resection of the abdominal aortic artery in rodents after application of permanent and transient metal clips for a period of 2 min, 5 min, 10 min, and 15 min in addition to the control. During the macroscopic examination of the fresh samples, cuts were made at the level of the transition between the clips, separated into six (n: 6) groups (transient metal clips of 2 min, 5 min, 10 min, 15 min, and the permanent metal clips and control) and subsequently fixed in buffered formaldehyde. The samples were processed in an automated manner and carefully included in paraffin for their correct orientation.
Histological cuts were made with a rotary microtome at 3 μm of thickness with routine histological technique (H&E) and special histochemical techniques (Masson’s Trichrome and orcein). During the microscopic examination, the traumatic alterations produced in the arterial wall were evaluated and documented through microphotographs.
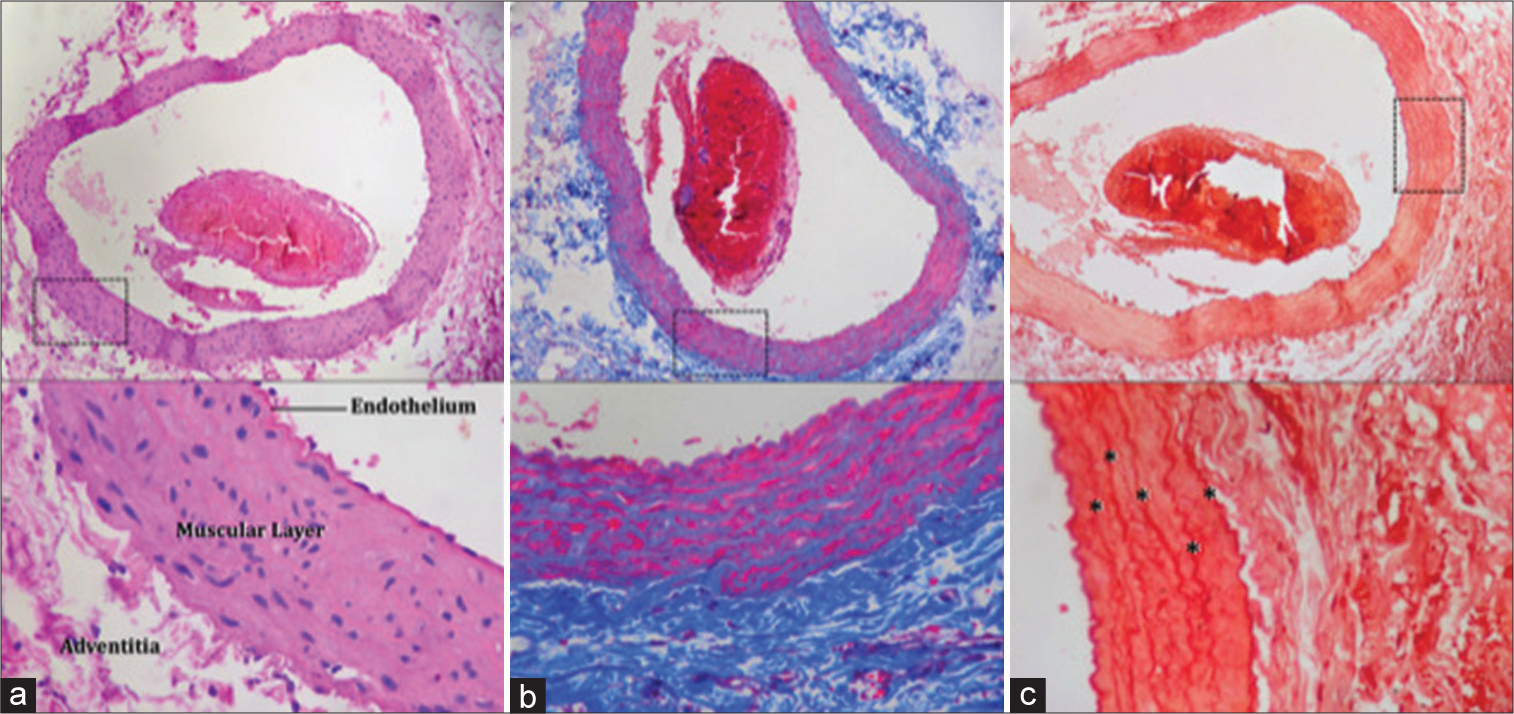
In Group I of the control sample, the integrity of the tissues is evidenced, with tunica adventitia, tunica media, and tunica intima without thickening or evidence of fibrinoid deposits. [
Figure 3:
Control Group I. (a) × 10 magnification with H&E staining: adventitia, middle tunic, and intimate tunic are observed without thickening of the layers, vessel wall with blood content and connective tissue. (b) × 10 magnification with Masson’s Trichrome staining: the vessel wall is observed, tunica adventitia, tunica media, and tunica intima without thickening, with evidence in blue of normal elastic fibers without thickening them. (c) × 10 magnification with Orcein stain: fragmentation of the blood vessel and marking of the elastic lamina with asterisks is observed. Dotted squares show an approach to the tissue shown in the figures.
Figure 4:
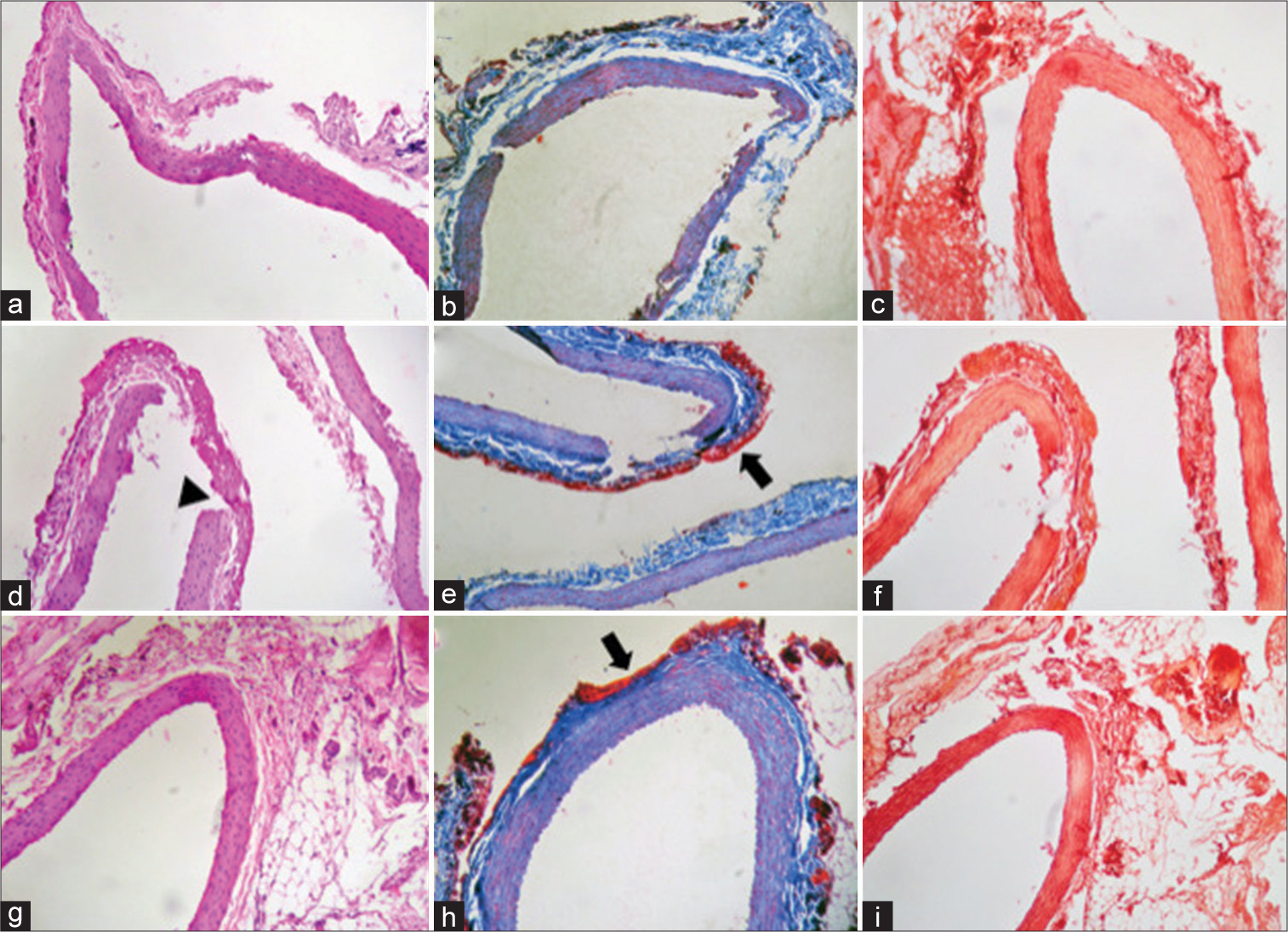
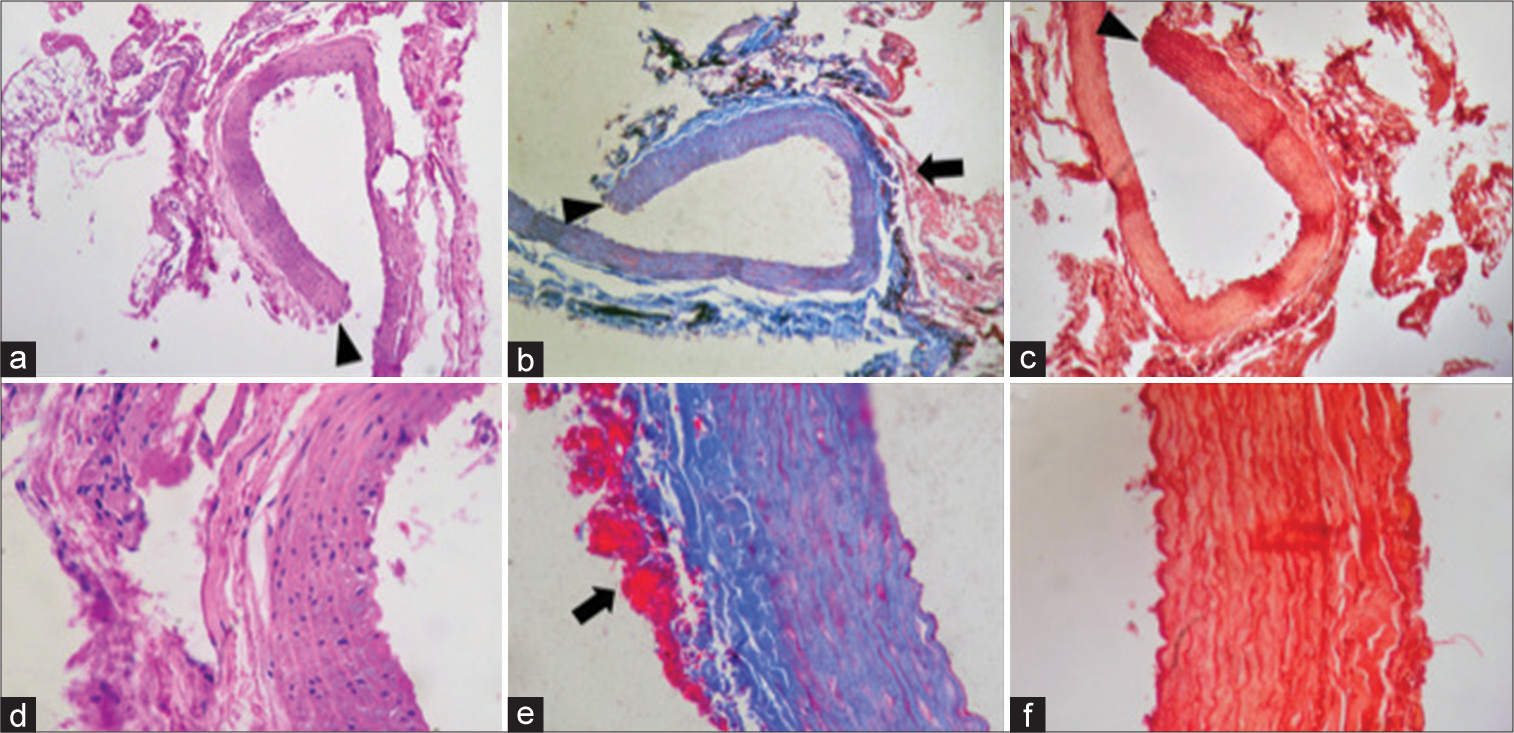
2, 5, and 10 min Clipping. (a-c) 2-min clipping (a) 10x magnification of H&E staining: tunica adventitia, tunica media, and tunica intima are observed without thickening of the layers, connective tissue with fragmentation of the wall without fibrin deposit in the tunica adventitia. (b) × 10 magnification of Masson’s Trichrome staining: the vessel wall is observed, tunica adventitia, tunica media, and tunica intima without thickening. (c) × 10 magnification of Orcein staining: no changes or rupture of the elastic lamina are observed. (d-f) Clip of 5 min. (d) × 10 magnification of H&E staining: transmural rupture secondary to the mechanical trauma of the clip is observed, which presents a different morphology marked with an arrowhead image. Scarce fibrinoid deposit at the site of rupture, with no data of inflammatory infiltrate or microhemorrhage. (e) × 10 magnification of Masson’s Trichrome staining: fibrin deposit marked with arrow. (f) × 10 magnification of Orcein staining: deposit observed with transmural rupture secondary to clip trauma. Separation of the muscle lamina and the fibrin layer unrelated to the time of clipping. (g-i) 10-min clipping (g) × 10 magnification of H&E staining: blood vessel without significant changes. (h) × 10 magnification of Masson’s Trichrome staining: no evidence of transmural rupture but with presence of fibrin deposit (in red). The arrow signs fibrin deposits. (i) × 10 magnification of Orcein staining: elastic lamina without thickening or changes.
Figure 5:
15-min CLIPPING. (a) × 10 magnification of H&E staining: transmural rupture of the vascular wall is observed with increased uptake of staining by evidence of fibrinoid deposit in the adventitia layer. Arrowhead points out in the transmural rupture. (b) × 10 magnification of Masson’s Trichrome staining: parietal rupture with fibrin deposit. Arrowhead transmural rupture and arrow fibrin deposits. (c) × 10 magnification of Orcein staining: transmural rupture is observed. Arrowhead point out parietal rupture. (d) × 40 magnification of H&E staining: marked fibrin deposit in the adventitia layer. (e) × 40 magnification of H&E staining: integrity of the blood vessel with fibrin deposit (in red). Arrow point out fibrin deposits. (f) × 40 magnification of Orcein staining: elastic lamina are stained, but without thickening or significant changes in them.
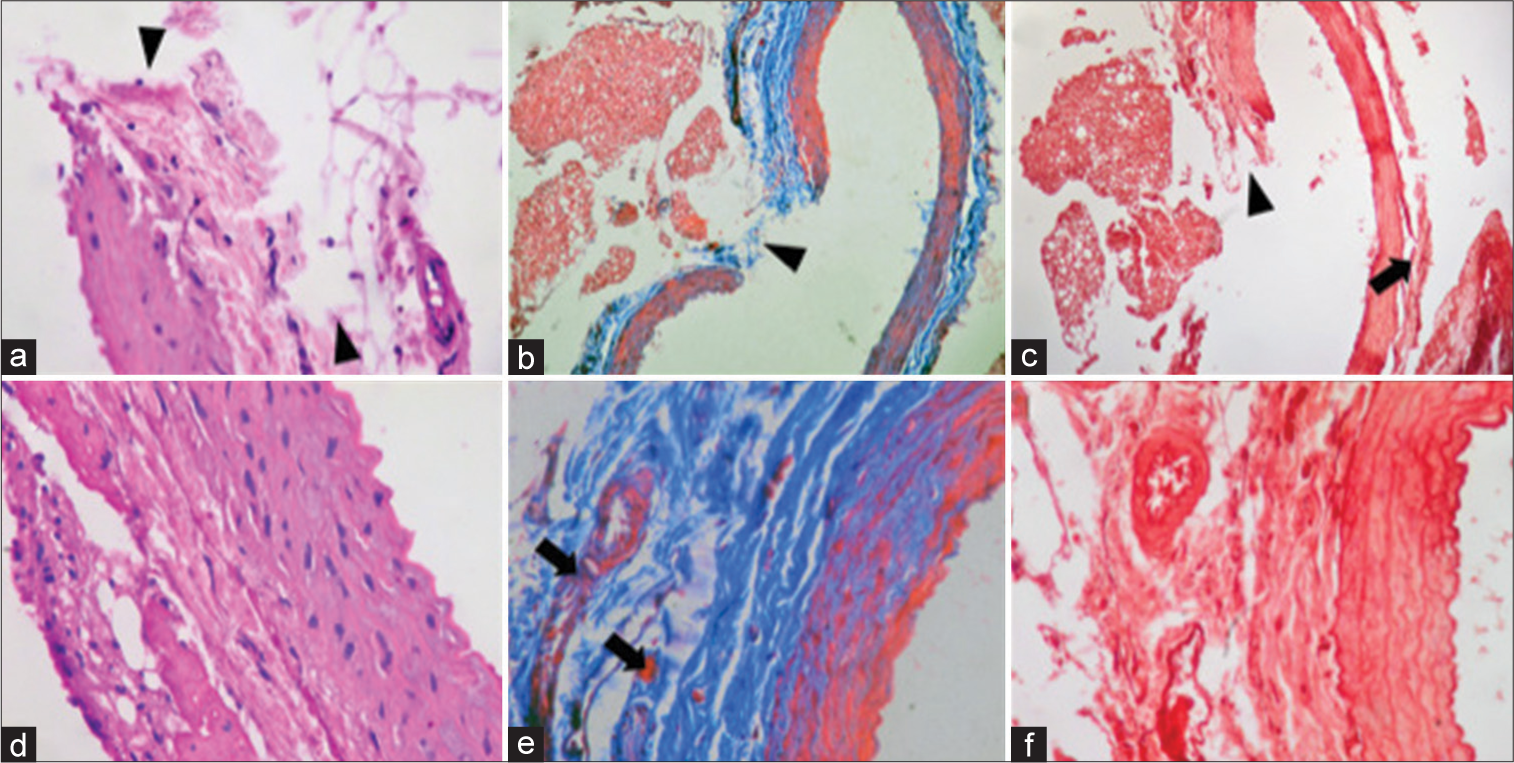
Figure 6:
Permanent clipping. (a) × 40 magnification of H&E staining: site of rupture of the entire vascular wall, observing continuity solution in the three layers with changes in morphology compared to the ruptures of 2, 5, and 10 min (arrowhead). (b) × 10 magnification of Masson’s Trichrome staining: continuity solution of the vascular wall is evidenced, with abundant fibrinoid deposit. Arrowhead point out with solution of continuity and fibrin deposit. (c) × 10 magnification of Orcein staining: continuity solution in the entire vessel wall (arrowhead) and rupture of the muscle lamina in the lateral region of the vessel (black arrow). (d) × 40 magnification of H&E staining: fibrin deposit is observed in the adventitia without thickening of the other layers. (e) × 40 magnification of Masson’s Trichrome staining: disruption of the elastic lamina with fibrin deposit in the adventitia layer (black arrow). (f) Increase × 40 Orcein staining: elastic lamina are evidenced, without changes or thickening, only the fibrin deposit of adventitia.
In none of the examined groups were endothelial lesions or subendothelial thickening identified exclusively, nor fragmentation of the tunica media or elastic lamina exclusively. With regards to the observed findings, transmural parietal rupture with associated parietal folding and fibrin deposits was identified in the groups that had permanent and transient clippings for 15 min, so it is concluded that the parietal damage is intimately related to the strength of the clip and the length of time, it remains in place. However, this study did not analyze parietal alterations of long-term transient or permanent clippings.
DISCUSSION
Since Cushing’s first description in 1911, vascular clips have become a necessary part of modern neurosurgery. Mc Kenzie in 1927 and Duane in 1950 made major changes to their material and shape. In 1953, Drew designed a special clip applicator that is still widely used today. In 1953, Norlen and Olivecrona developed a wire clip that could be reopened by applying support to the rear axis of the clip. In 1969, Heifetz designed a clip with an internal wire spring which reduced the fatigue of the clip. In 1971, Mayfield and Kees improved the design of Schwartz’s cross-leaf clip, and the resulting Mayfield clip with silicone-covered tips was the most widely used spring clip for some time.[
In 1928, Pool reported that Jefferson performed the first provisional ligation of the proximal artery, while Gibbs in 1957 during neck dissection of an aneurysm performed a temporary ligation using a modified Cairns clip. In 1969, Suzuki et al. measured for the first time, in 215 patients with aneurysms, the maximum safe time limit for cerebral ischemia when performing a temporary clipping with moderate hypothermia. In 1983, Bae et al. reported that temporary clipping of the middle cerebral artery in 16 patients could withstand up to 20 min to minimize complications.[
Dujovny et al. demonstrated four levels of damage in the endothelial wall after temporary clipping: 1. crushing, 2. disruption, 3. disruption with clot formation, and 4. fracture with exposure of the tunica media, directly linked to the closing force of the vascular clip,[
On the other hand, Gertz et al. in 1976, in a study of 25 white rabbits where they worked with temporary clipping for 5, 15, and 30 min as well as 2 h, found that the carotid arteries that were occluded did not macroscopically exhibit any changes, although on examination under scanning and transmission electron microscopy on the compression surface, variation in the severity of lesions was observed, from distortions of the endothelial surface of the vessel, up to cell fragmentation that compromised endothelial continuity with exposure of the subendothelial connective tissue. These alterations were detected in arterial segments that were clipped for at least 5 min; however, they did not change in frequency or increase in severity with long clippings of 30 min or 2 h,[
As far as the effect of the clipping time, Dodson et al. worked on animals where the artery was occluded for half hour and 1 h, showing that the changes were more prominent in proximal clipping areas rather than in distal ones, consistent with myonecrosis. It was concluded that the initial alterations were induced by the production of chemicals by nerve damage and stasis of vascular elements and/or direct trauma to the wall.[
Koh and Kwon in 2007, in an experimental study of 25 rats, which underwent continuous and intermittent temporal clipping, observed significant changes in the vessel wall with continuous clipping above 5 min, reporting moderate endothelial cell damage, as well as disruption of adventitia at 10 min of continuous clipping and loss of endothelial cells and segmental damage of muscle fibers at 15 and 20 min.[
In 1982, Ebina et al. performed 17 postsurgical autopsies of permanent clippings for a period of time of 6 days– 11 months, where it is observed that the granulation tissue around the vascular clip occurred beyond 1 month, the aneurysm lumen was also observed in cases beyond 13 days of clipping, showing formation of mural thrombus and fibrin deposit, as well as thickening of the tunica intima. After 1 month, the thickening of the tunica intima was fuzzy and it had organized thrombus, while at 3 months or more, the lumen of the aneurysm was almost completely closed due to thickening of the tunica intima, which could not be evaluated over time in our work.[
Zhou et al. in 1993 have already mentioned that with regards to temporary arterial occlusion and the risk of damage to the blood vessel wall, the relationship between damage to the arterial wall, the contact area of the clip, the closing pressure of the clip, the diameter of the blood vessel, the elasticity of the blood vessel, the blood pressure and the time of occlusion was well known.[
Hoff and Gottlob stated that even transient clipping caused an abnormal overlap of endothelial cells and the formation of pseudopods of smooth muscle cells that were projected into the light, which were reversible changes. The irreversible changes were necrosis and thickening of the intima cells, retention of fibrin, cholesterol, etc. in the subendothelial space and fragmentation of the elastic lamina.[
Limitations
One of the limitations of this work is the vascular anatomical variability of our experimental model in comparison to that of the human species, since a difference was observed in the muscle layer of Wistar rats, which has multiple concentric elastic lamina, while the intracerebral blood vessels of humans have an internal and external muscle layer.
Another pending aspect to be analyzed is the effect of transient and permanent long-term clipping and observation is required of the postsurgical effect of clipping over time with monthly monitoring, to evaluate if there is remodeling of the vessel or exacerbation of histological damage.
We believe that this type of experimental work motivates the future studies, which should examine in detail if the histological damage is directly related to the trauma of the clipping’s time and closing force or if it is the result of sustained ischemia of the vasa vasorum.
CONCLUSION
Based on the series of papers published to date, whose results coincided with our findings, the histological vascular effect after both transient and permanent clipping is indisputable, which makes it essential to understand the effect of the clipping force as well as its application time on the vascular endothelium.
It is concluded that the sum of time and force in the clipping application induces objectifiable vascular changes after 5 min, which is why it would be recommendable to avoid exceeding this length of transient clipping time and reduce the possibility of rearranging the permanent clip in the same site where it has been placed.
Declaration of patient consent
The authors certify that they have obtained all appropriate consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bae J, Kim K, Ko Y, Oh SJ, Oh SH. An experimental study for the effect of interrupted (intermittently-repeated) versus continuous temporary clipping on the change of arterial wall of rat. Korean J Cerebrovasc Surg. 2000. 2: 5-10
2. Castillo-Rangel C, Marín G, Hernandez-Contreras KA, ZarateCalderon C, Vichi-Ramirez MM, Cortez-Saldias W. Atlas of nervous system vascular malformations: A systematic review. Life. 2022. 12: 1199
3. Castillo-Rangel C, Salinas-Velázquez O, Gomez-Ibarra A, Becerra-Escobedo G, Pérez VH, Marín-Márquez G. Report of an epicranial arteriovenous malformation. Cesk Slov Neurol. 2021. 84: 488-90
4. D’Souza S. Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2015. 27: 222-40
5. Dodson RF, Tagashira Y, Chu LW. Acute ultrastructural changes in the middle cerebral artery due to the injury and ischemia of surgical clamping. Can J Neurol Sci. 1976. 3: 23-7
6. Dujovny M, Munoz G, Nelson D, Langhi R, Fein JM, Ba NK. Reduced vascular trauma after temporary occlusion with modified biemer and yasargil clips. Microsurgery. 1981. 2: 195-201
7. Ebina K, Iwabuchi T, Suzuki S. Histological change in permanently clipped or ligated cerebral arterial wall. Acta Neurochirurgica. 1982. 66: 23-42
8. Gertz SD, Rennels ML, Forbes MS, Kawamura J, Sunaga T, Nelson E. Endothelial cell damage by temporary arterial occlusion with surgical clips. J Neurosurg. 1976. 45: 514-9
9. Hoff HF, Gottlob R. Ultrastructural changes of large rabbit blood vessels following mild mechanical trauma. Virchows Arch Path Anat. 1968. 345: 93-106
10. Koh HS, Kwon OY. Experimental study on the effect of temporary clipping on the histological changes of the arterial walls of rats. J Korean Soc Biomed Sci. 2007. 13: 111-7
11. Liang-fu Z, Shi-qi L, Mu-long Y. Temporary Arterial occlusion during intracranial aneurysm surgery. Chin Med J. 1993. 106: 803-8
12. Rangel CC, Cordova JS, Noriega AR, Sanchez JM, Frías AA, Pradel RA. Foramen magnum meningioma: Surgical planning analysis with 3d printing. J Neuro Brain Res. 2021. 1: 1-4
13. Richling B, Griesmayr G, Lametschwandtner A, Scheiblbrandner W. Endothelial lesions after temporary clipping. A comparative study. J Neurosurg. 1979. 51: 654-61
14. Van Lieshout JH, Dibué-Adjei M, Cornelius JF, Slotty PJ, Schneider T, Restin T. An introduction to the pathophysiology of aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2018. 41: 917-30
15. Zheng VZ, Wong GK. Neuroinflammation reponses after subarachnoid hemorrhage: A review. J Clin Neurosci. 2017. 42: 7-11