- Department of Surgery, Division of Neurosurgery, The Ottawa Hospital, University of Ottawa, Civic Campus, Ottawa, ON, Canada,
- Department of Neurosurgery, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts,
- Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States,
- Department of Pathology, King Fahad Medical City, Riyadh, Saudi Arabia
- Department of Neurosurgery, King Saud University, Riyadh, Saudi Arabia
- Department of Basic Sciences, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- Division of Neurosurgery, Department of Surgery, College of Medicine, King Abduaziz University, Jeddah, Saudi Arabia,
- Clinical Skills and Simulation Center, King Abduaziz University, Jeddah, Saudi Arabia.
Correspondence Address:
Mohammad M. Alshardan
Division of Neurosurgery, Department of Surgery, College of Medicine, King Abduaziz University, Jeddah, Saudi Arabia,
Clinical Skills and Simulation Center, King Abduaziz University, Jeddah, Saudi Arabia.
DOI:10.25259/SNI_26_2020
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mohammad M. Alshardan1, Abdullah M. Abunimer2, Hussam Abou-Al-Shaar3, Sadeq Aldandan4, Sherif M. El-Watidy5, Ali M. Mustafa6, Abdulrahman J. Sabbagh7,8. Histopathological changes of neuronal tissue following the use of hydrogen peroxide in neurosurgical procedures. 08-Mar-2021;12:91
How to cite this URL: Mohammad M. Alshardan1, Abdullah M. Abunimer2, Hussam Abou-Al-Shaar3, Sadeq Aldandan4, Sherif M. El-Watidy5, Ali M. Mustafa6, Abdulrahman J. Sabbagh7,8. Histopathological changes of neuronal tissue following the use of hydrogen peroxide in neurosurgical procedures. 08-Mar-2021;12:91. Available from: https://surgicalneurologyint.com/surgicalint-articles/10632/
Abstract
Background: Hydrogen peroxide (HP) is routinely used in neurosurgical procedures to achieve surgical hemostasis. However, its safety profile is still debatable with various reports depicting range of adverse effects on neuronal tissue. The objective of this paper is to evaluate the safety and efficacy of HP as a hemostatic agent in normal neuronal tissue during neurosurgical procedures conducted on rats.
Methods: One hundred rats were divided into three groups. The first and third group underwent cortical irrigation with HP and the second group underwent spinal irrigation with HP. All groups were irrigated with different concentrations of HP (1%, 3%, or 6%) for 3 min and tissue biopsies were obtained immediately afterwards (Groups A and B) or 1 week after HP irrigation (Group C). Study specimens were examined histologically and compared to control tissue.
Results: All rats showed normal behavioral, functional, and motor neurological activity following the procedures. Histopathologically, dark neurons were observed in all HP exposed tissue. The cytoplasm revealed condensed and dark Nissl substance and the neurites and axons exhibited a corkscrew morphology. No ischemic changes or inflammatory infiltrates were detected. The majority of dark neurons were observed at the periphery of tissue fragments. These findings were present and consistent in both the short- and long-term groups.
Conclusion: HP irrigation showed no significant short- or long-term clinical and histopathological changes in comparison to normal saline when used on rats’ neuronal tissue. This may confirm the safety of intraoperative HP usage as hemostatic agent during neurosurgical procedures.
Keywords: Brain, Hemostasis, Hydrogen peroxide, Intracerebral hemorrhage, Spinal cord
INTRODUCTION
Achieving hemostasis in neurosurgery is considered a key element to prevent postoperative complications and the need for reoperation. Various methods have been utilized to achieve this goal including thermal, mechanical, and chemical hemostasis.[
Although the use of HP results in favorable hemostatic outcomes, its potential deleterious effects on neuronal tissue is not fully investigated. The objective of this study was to evaluate the safety and efficacy of HP as a hemostatic agent in neurosurgical procedures by examining the clinical and histopathological changes of normal rats’ neuronal tissue following the exposure to different HP concentrations.
MATERIALS AND METHODS
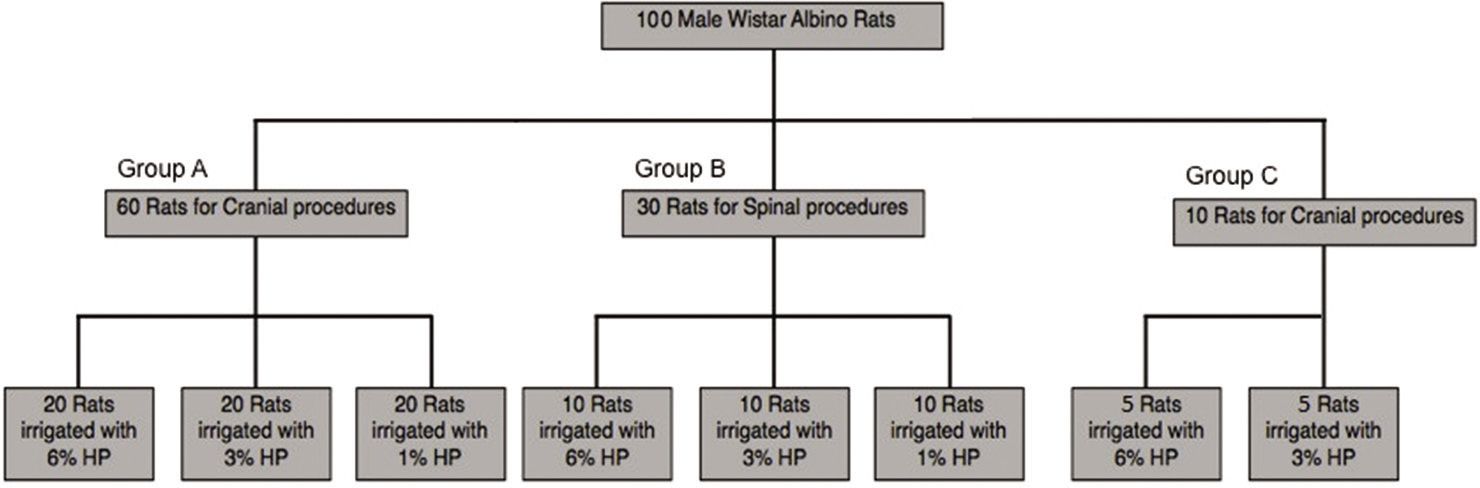
All animal procedures were approved by King Saud University College of Medicine Animal Care and Use Committee and the Institutional Review Board Committee at King Fahad Medical City (Riyadh, Saudi Arabia). A total of 100 male Wistar albino rats weighing 250 g–300 g were obtained and divided into three groups (Groups A, B, and C). Rats’ age, sex, species, and weight were similar among the groups. Group A included 60 rats and was further subdivided into three groups with 20 rats each. Group B included 30 rats and was further subdivided into three groups with ten rats each. Group C included ten rats and was further subdivided into two groups with five rats each [
Figure 1:
Distribution of the different concentration of hydrogen peroxide (HP) among the experimental groups. Group A (n = 60) underwent cranial procedures and received irrigation of 1%, 3%, and 6% HP. Group B (n = 30) underwent spinal procedures and received irrigation of 1%, 3%, and 6% HP. Group C (n = 10) received an intracranial irrigation of 3% and 6% HP and was sampled after 1 week to evaluate the long-term effects of HP.
Each animal was anesthetized using pentobarbitone (50 mg/kg body weight) before the neurosurgical procedure. Rats in Groups A and C underwent cranial procedures while those in Group B underwent spinal procedures. Groups A and B rats received irrigation with normal saline (control specimen) and one of three (1%, 3%, or 6%) HP concentrations (study specimen) for 3 min [
Control tissue was obtained from the same rat with either interhemispheric brain or interdistal spinal cord tissue following irrigation with normal saline for 3 min. Group C animals were monitored and clinically assessed for any neurological change for 1 week following the use of HP.
Cranial procedures
Skulls of Group A rats were exposed using a longitudinal incision in the midline after an infiltration with 2% lidocaine with epinephrine; a right unilateral craniotomy was then performed using a manual drill (Stoelting Co., IL). The dura was then opened with a number ten blade scalpel. A small corticectomy was performed and the exposed brain tissue was irrigated with 0.9% normal saline for 3 min, and a control brain specimen was obtained and placed in 10% formalin for fixation. Another craniotomy on the left side was performed in the same fashion as the right side. A small corticectomy was performed and the brain tissue was irrigated with one of three HP concentrations (1%, 3%, or 6%) for 3 min followed by irrigation with normal saline and suctioning. Study specimens were collected from the irrigated area. Animals were sacrificed immediately thereafter with an overdose of pentobarbital.
Spinal procedures
The spine was exposed using a longitudinal incision in the midline and laminectomies were performed in two non-contiguous levels (upper cervical and lower thoracic). Cottonoid at the caudal end of the cervical laminectomies and at the rostral end of the lower thoracic laminectomies were placed to avoid contamination and cross spillage. A small incision in the spinal cord was made in both areas and the upper levels were irrigated with normal saline for 3 min, while the lower levels were irrigated with one of three HP concentrations (1%, 3%, or 6%) for 3 min followed by irrigation with normal saline and suctioning. Spinal cord tissue and dura specimens were collected from the exposed upper levels (control specimen) and lower levels (HP specimen). Animals were sacrificed immediately thereafter with an overdose of pentobarbital.
Cranial procedures for long-term effect
The rats in Group C were positioned in a stereotaxic frame (Stoelting Co., IL). In a sterile field, a midline scalp incision was made, and the underlying muscles were removed. A unilateral burr hole was drilled. A 10 μl Hamilton microsyringe was used to irrigate 3% or 6% HP. The syringe was mounted on a syringe holder and fixed on the stereotaxic frame. The needle was lowered to exactly 5.0 mm below the surface of the skull. A volume of 5 μl of 3% or 6% HP according to the subgroup was irrigated slowly at a rate of 1 μl/min. The needle was slowly removed to prevent the injected fluid from escaping the needle tract. All rats received prophylactic antibiotics. The skin incision was sutured using 3–0 silk suture. The rats were kept under observation for 1 week. After 1 week, the rats were anesthetized in a similar fashion as described previously. The first burr hole was opened, and a study specimen was obtained. Adjacent to the site of the first hole, another burr hole was made, and control brain tissue was obtained and stored in 10% buffered formalin for evaluation. Animals were sacrificed immediately thereafter with an overdose of pentobarbital.
Tissue sampling and processing
Two hundred samples were sent to an independent neuropathologist for evaluation. Perfusion, tissue removal, fixation, sectioning, and mounting on glass slides were performed as previously described.[
Statistical analysis
Statistical analysis was performed using one-way ANOVA and Duncan’s method for pairwise multiple comparisons. Data are expressed as the mean ± SEM. Statistical analysis was performed using SPSS version 17.0 (IBM Corporation, USA).
RESULTS
Clinical outcomes
During the 1-week monitoring of Group C rats, all animals showed normal behavioral, functional, and motor neurological activity with no neurological deficits following HP application.
Histopathological findings
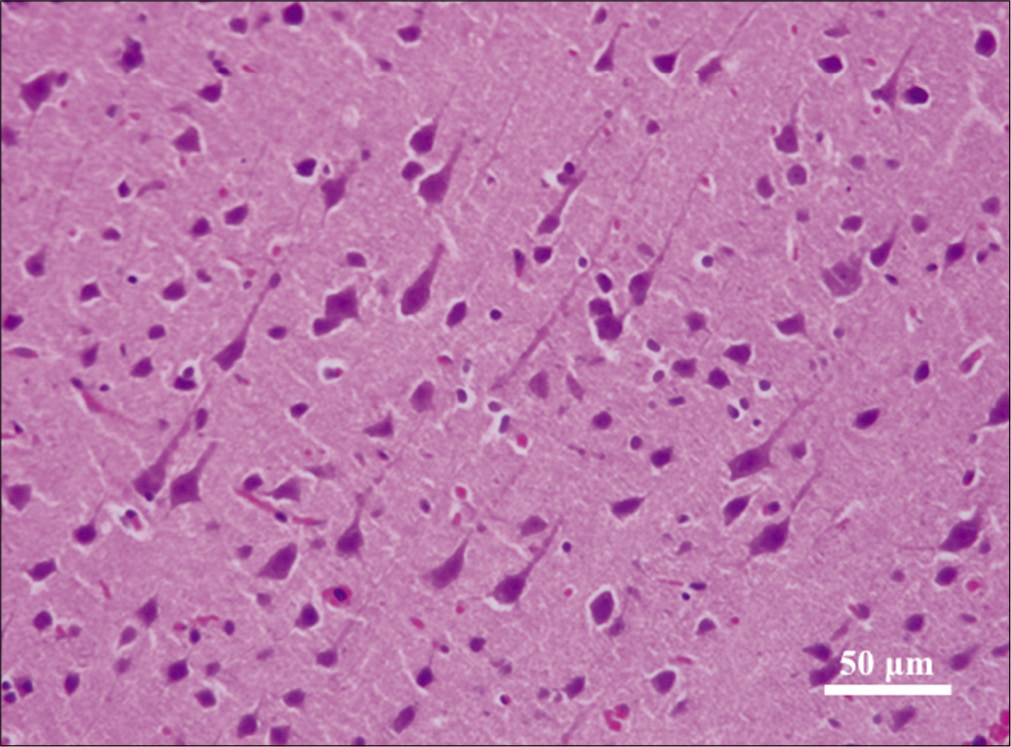
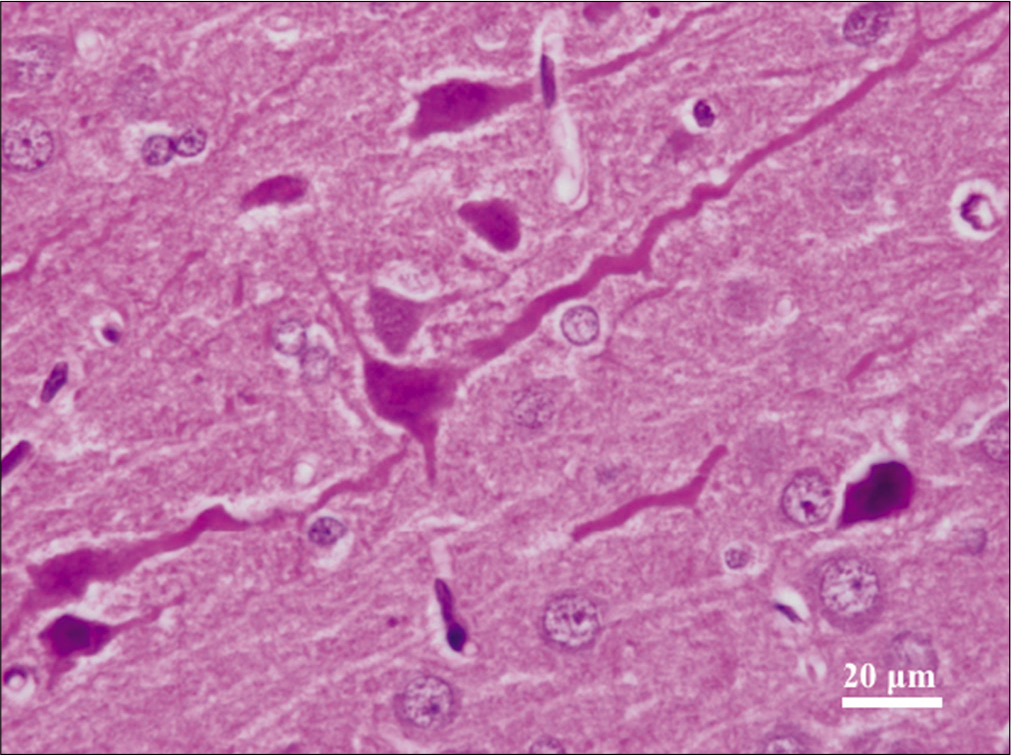
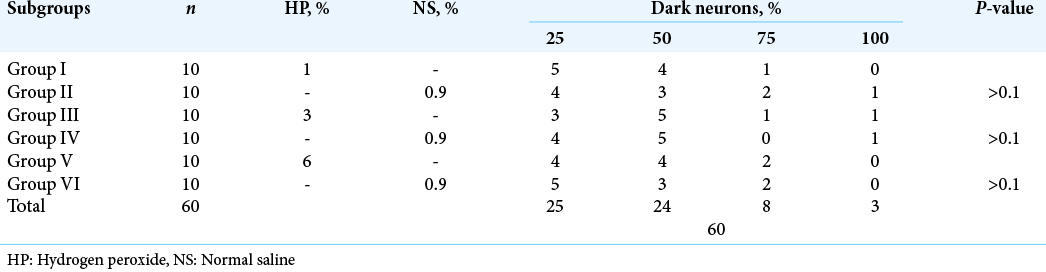
Across both cranial and spinal specimens, the cytoplasm revealed condensed and dark Nissl substance. The neurites and axons exhibited corkscrew morphology [
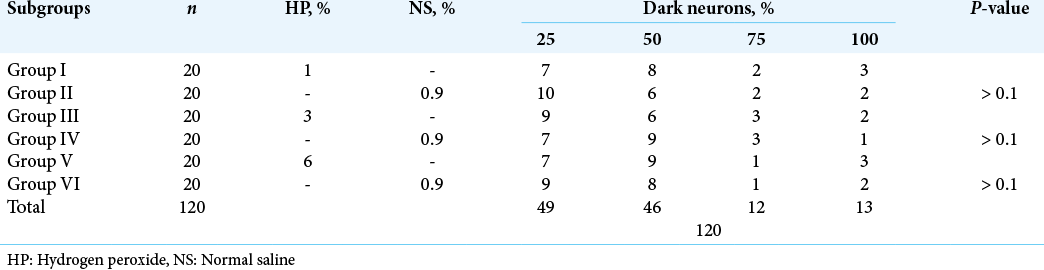
The dark neurons were present in nearly equal percentages in the experimental and control tissues [
DISCUSSION
HP is routinely utilized during cranial and spinal cord surgeries to achieve hemostasis.[
Despite its evident efficacy, HP safety remains debatable. To the best of our knowledge, HP’s safety has been evaluated in at least six studies, in which histopathological changes were assessed following the use of HP on neural tissue.[
On the contrary, none of the aforementioned changes were evident in the rats’ brain and spinal cord tissue in our study. In all 100 rats, only dark neurons could be observed in both experimental and control groups with no statistically significant difference between the three groups, or between the cranial and spinal groups. The histopathological changes were similar in the short-term groups (A and B) and long-term group (C) with no signs of ischemia, inflammation, or necrosis in all groups. The prevalence of dark neurons at the periphery of the tissue can be attributed to the strong and rapid formaldehyde penetration at the edge of the tissue or to the death of neurons in the center of the examined specimen, which precludes the formation of dark neurons. In addition, the prevalence of dark neurons might be attributed to the difference in exposure time between our study and the reported studies (3 min vs. 5 min), which may affect the occurrence of neuronal cell death, the different species (rats vs. cats) studied, or the histopathological interpretation of dark neurons. Although our findings are inconsistent with the results of previous studies, they are consistent with the ongoing clinical practice and observed patients’ outcomes.
Various in vitro studies have also observed tumoricidal activity of HP on different types of cancer cells.[
In addition to its hemostatic and antitumor activities, HP is a potent oxidizing reagent and thus, it is commonly used to disinfect wounds against a wide range of microorganisms. As HP contacts tissues, it generates reactive oxygen species such as singlet oxygen and hydroxyl radicals that react with membrane lipids and bacterial DNA.[
In clinical settings, Lichtenbaum et al.[
Oxygen embolization is the most commonly reported complication associated with HP use with less than 10 cases reported in the literature.[
Limitations
Our study is limited by the lack of assessment of meningothelial cells, leptomeningeal vasculature, and blood-brain barrier changes following HP application as well as the lack of other measures in assessing neuronal response such as immunofluorescence testing and immunohistochemical and nuclear stains. We did not perform an extensive histopathological examination to the wounded surfaces as well. In addition, the study is limited by the lack of standardized measurements of behavioral, functional, and motor neurological activity of the rats in prolonged application group (Group C). However, our study demonstrates that HP safety is comparable to that of normal saline when applied carefully to the brain and spinal cord surfaces.
CONCLUSION
Intraoperative usage of HP is potentially safe and effective modality to achieve hemostasis in neurosurgical procedures. Our study demonstrated the absence of significant short- and long-term clinical and histopathological changes following HP application in comparison to normal saline in rats’ brain and spinal cord tissue.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arand AG, Sawaya R. Intraoperative chemical hemostasis in neurosurgery. Neurosurgery. 1986. 18: 223-33
2. Dauch WA. Infection of the intervertebral space following conventional and microsurgical operation on the herniated lumbar intervertebral disc. A controlled clinical trial. Acta Neurochir (Wien). 1986. 82: 43-9
3. Dolan EJ. Danger of use of hydrogen peroxide in stereotactic biopsies. Appl Neurophysiol. 1987. 50: 237-8
4. Dubey PK, Singh AK. Venous oxygen embolism due to hydrogen peroxide irrigation during posterior fossa surgery. J Neurosurg Anesthesiol. 2000. 12: 54-6
5. Epstein JA. Hydrogen peroxide for hemostasis. Neurosurgery. 1987. 20: 63
6. Gruber RP, Vistnes L, Pardoe R. The effect of commonly used antiseptics on wound healing. Plast Reconstr Surg. 1975. 55: 472-6
7. Hankin FM, Campbell SE, Goldstein SA, Matthews LS. Hydrogen peroxide as a topical hemostatic agent. Clin Orthop Relat Res. 1984. p. 244-8
8. Hansson E, Vallfors B. A study of irrigation fluids for neurosurgery on brain primary cell cultures. Experientia. 1980. 36: 64-5
9. Huang C, Pik J. Tension pneumocephalus and oxygen emboli from hydrogen peroxide irrigation. J Clin Neurosci. 2014. 21: 323-5
10. Jaques LB, Bell HJ. The reduction of hydrogen peroxide by fibrin. Can J Res. 1946. 24: 79-83
11. Kwon D, Choi C, Lee J, Kim KO, Kim JD, Kim SJ. Hydrogen peroxide triggers the expression of Fas/FasL in astrocytoma cell lines and augments apoptosis. J Neuroimmunol. 2001. 113: 1-9
12. Lichtenbaum R, de Souza AA, Jafar JJ. Intratumoral hydrogen peroxide injection during meningioma resection. Neurosurgery. 2006. 59: ONS470-3
13. Lopez LM, Traves N, Napal M. Fatal gas embolism during corrective surgery for scoliosis using the posterior approach. Rev Esp Anestesiol Reanim. 1999. 46: 267-70
14. Mawk JR. Hydrogen peroxide for hemostasis. Neurosurgery. 1986. 18: 827
15. Mesiwala AH, Farrell L, Santiago P, Ghatan S, Silbergeld DL. The effects of hydrogen peroxide on brain and brain tumors. Surg Neurol. 2003. 59: 398-407
16. Morikawa H, Mima H, Fujita H, Mishima S. Oxygen embolism due to hydrogen peroxide irrigation during cervical spinal surgery. Can J Anaesth. 1995. 42: 231-3
17. Mut M, Yemisci M, Gursoy-Ozdemir Y, Ture U. Hydrogen peroxide-induced stroke: Elucidation of the mechanism in vivo. J Neurosurg. 2009. 110: 94-100
18. Nicholson NC, Ramp WK, Kneisl JS, Kaysinger KK. Hydrogen peroxide inhibits giant cell tumor and osteoblast metabolism in vitro. Clin Orthop Relat Res. 1998. 347: 250-60
19. Prabhakar H, Rath GP, Dash HH. Bradycardia following hydrogen peroxide irrigation during posterior fossa surgery. Anaesthesia. 2006. 61: 914
20. Prabhakar H, Rath GP. Venous oxygen embolism with use of hydrogen peroxide during craniotomy in the supine position. J Clin Neurosci. 2008. 15: 1072
21. Renkens KL, Payner TD, Leipzig TJ, Feuer H, Morone MA, Koers JM. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila Pa 1976). 2001. 26: 1645-50
22. Shah J, Pedemonte MS, Wilcock MM. Hydrogen peroxide may cause venous oxygen embolism. Anesthesiology. 1984. 61: 631-2
23. Spiriev T, Tzekov C, Kondoff S, Laleva L, Sandu N, Arasho B. Trigemino-cardiac reflex during chronic subdural haematoma removal: Report of chemical initiation of dural sensitization. JRSM Short Rep. 2011. 2: 27
24. Steinberg D, Heling I, Daniel I, Ginsburg I. Antibacterial synergistic effect of chlorhexidine and hydrogen peroxide against Streptococcus sobrinus, Streptococcus faecalis and Staphylococcus aureus. J Oral Rehabil. 1999. 26: 151-6
25. Ulivieri S, Toninelli S, Petrini C, Giorgio A, Oliveri G. Prevention of post-operative infections in spine surgery by wound irrigation with a solution of povidone-iodine and hydrogen peroxide. Arch Orthop Trauma Surg. 2011. 131: 1203-6
26. Vallfors B, Hansson HA, Belghmaidi M. Mesothelial cell integrity of the subdural and arachnoid surfaces of the cat brain after exposure to neurosurgical irrigation fluids and air: A scanning electron microscopic study. Neurosurgery. 1983. 12: 35-9
27. Vallfors B, Hansson HA, Svensson J. Experimental exposure of the cat brain surface to neurosurgical irrigation fluids and air: Study of blood-brain barrier damage. Neurosurgery. 1980. 7: 53-6
28. Vallfors B, Rosengren LE, Persson LI. Exposure of the cat brain surface to neurosurgical irrigation fluids, hydrogen peroxide and air. Quantitative assay of blood-brain barrier dysfunction. Acta Neurochir (Wien). 1982. 64: 225-32
29. Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci USA. 1997. 94: 12676-81
30. Zimmerman GA, Lipow KI. Pneumocephalus with neurological deficit from hydrogen peroxide irrigation. Case illustration. J Neurosurg. 2004. 100: 1122