- Departments of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
- Departments of Pathology Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
- Departments of Radiology, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
Correspondence Address:
Tatsuki Oyoshi
Departments of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan.
DOI:10.25259/SNI_531_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Madan Bajagain1, Tatsuki Oyoshi1, Tomoko Hanada1, Nayuta Higa1, Tsubasa Hiraki2, Kiyohisa Kamimura3, Shinichi Kuroki1, Koji Yoshimoto1. Histopathological variation in the demyelinating sentinel lesion of primary central nervous system lymphoma. 15-Oct-2020;11:342
How to cite this URL: Madan Bajagain1, Tatsuki Oyoshi1, Tomoko Hanada1, Nayuta Higa1, Tsubasa Hiraki2, Kiyohisa Kamimura3, Shinichi Kuroki1, Koji Yoshimoto1. Histopathological variation in the demyelinating sentinel lesion of primary central nervous system lymphoma. 15-Oct-2020;11:342. Available from: https://surgicalneurologyint.com/surgicalint-articles/10329/
Abstract
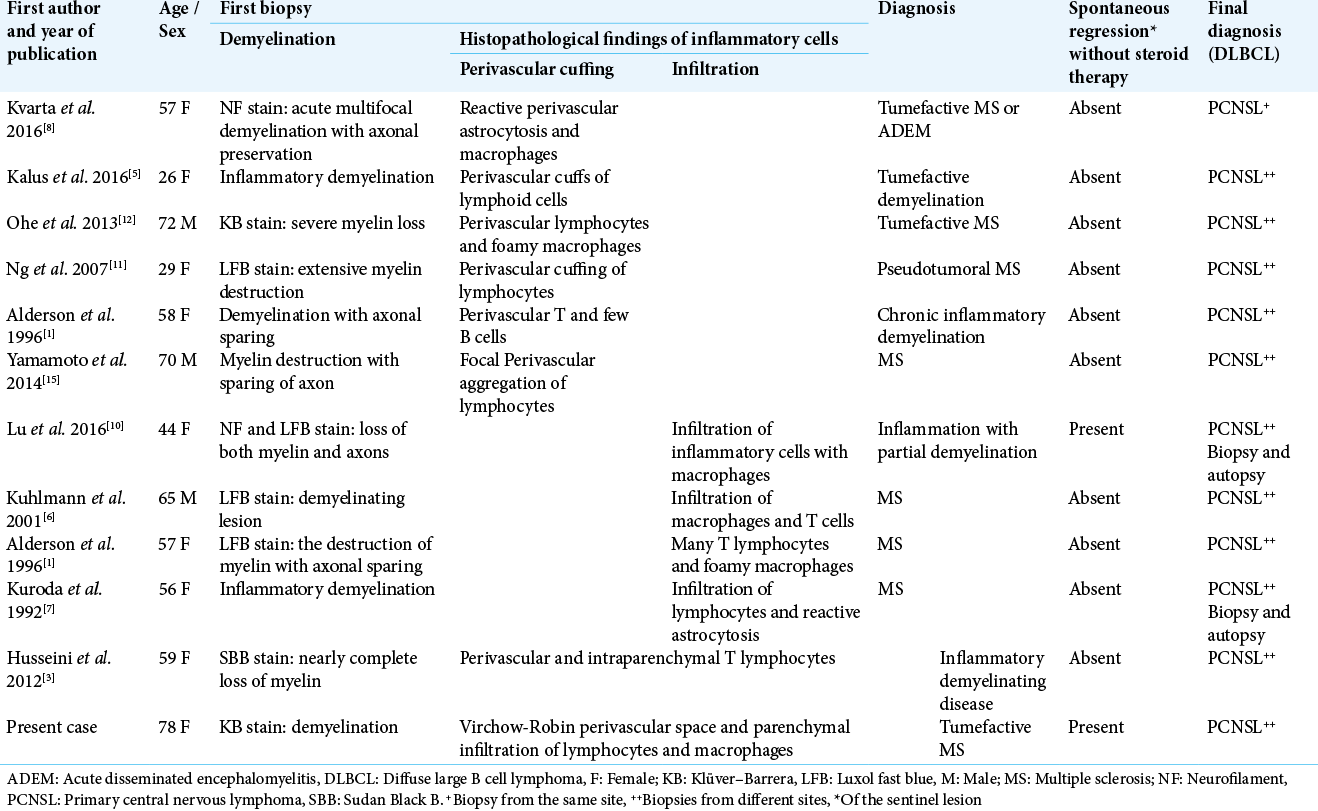
Background: Primary central nervous system lymphoma (PCNSL) is one of the least common malignant brain tumors. It is usually diagnosed initially as diffuse large B cell lymphoma (DLBCL). In rare cases, however, a demyelinating lesion referred to as a “sentinel lesion” precedes the actual diagnosis, which usually depicts two distinct patterns of inflammatory cells during histological analysis. This case report describes a unique histological finding and describes the recognized variations in sentinel lesion histopathology.
Case Description: A 78-year-old female patient was found to have multiple white matter lesions of various degrees of enhancement on post-contrast T1-weighted magnetic resonance imaging. A stereotactic biopsy of a heterogeneous lesion in the left occipital lobe was performed, which revealed demyelination along with lymphocytic infiltration, reactive astrocytosis, abundant T cells, and foamy macrophages. There was no evidence of monoclonality, rapid regression of all lesions occurred, and the patient was thus treated for tumefactive demyelination. Three months later, all of the residual lesions had enlarged and were homogeneously enhancing. An endoscopic-guided biopsy of the right periventricular lesion showed diffuse atypical lymphoid cells.
Conclusion: The sentinel lesion of PCNSL expresses a variable histological pattern of inflammatory cells. This case demonstrates a unique and rare picture of mixed perivascular and parenchymal infiltration of inflammatory cells, highlighting the importance of repeated biopsies and/or radiological examinations to obtain an accurate diagnosis.
Keywords: Central nervous system lymphoma, Demyelinating sentinel lesion, Parenchymal infiltration, Perivascular cuffing, Spontaneous regression
INTRODUCTION
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin’s lymphoma confined to the brain, leptomeninges, and the spinal cord.[
A review of the literature revealed only eleven cases of demyelinating sentinel lesions in PCNSL have been reported to date [
Here, we present the oldest patient with a completely demyelinating sentinel lesion reported to date, who had the distinct histology of combined perivascular and parenchymal infiltration of inflammatory cells (T lymphocytes and macrophages). The sentinel lesion we describe also had spontaneous regression without steroid therapy.
CASE PRESENTATION
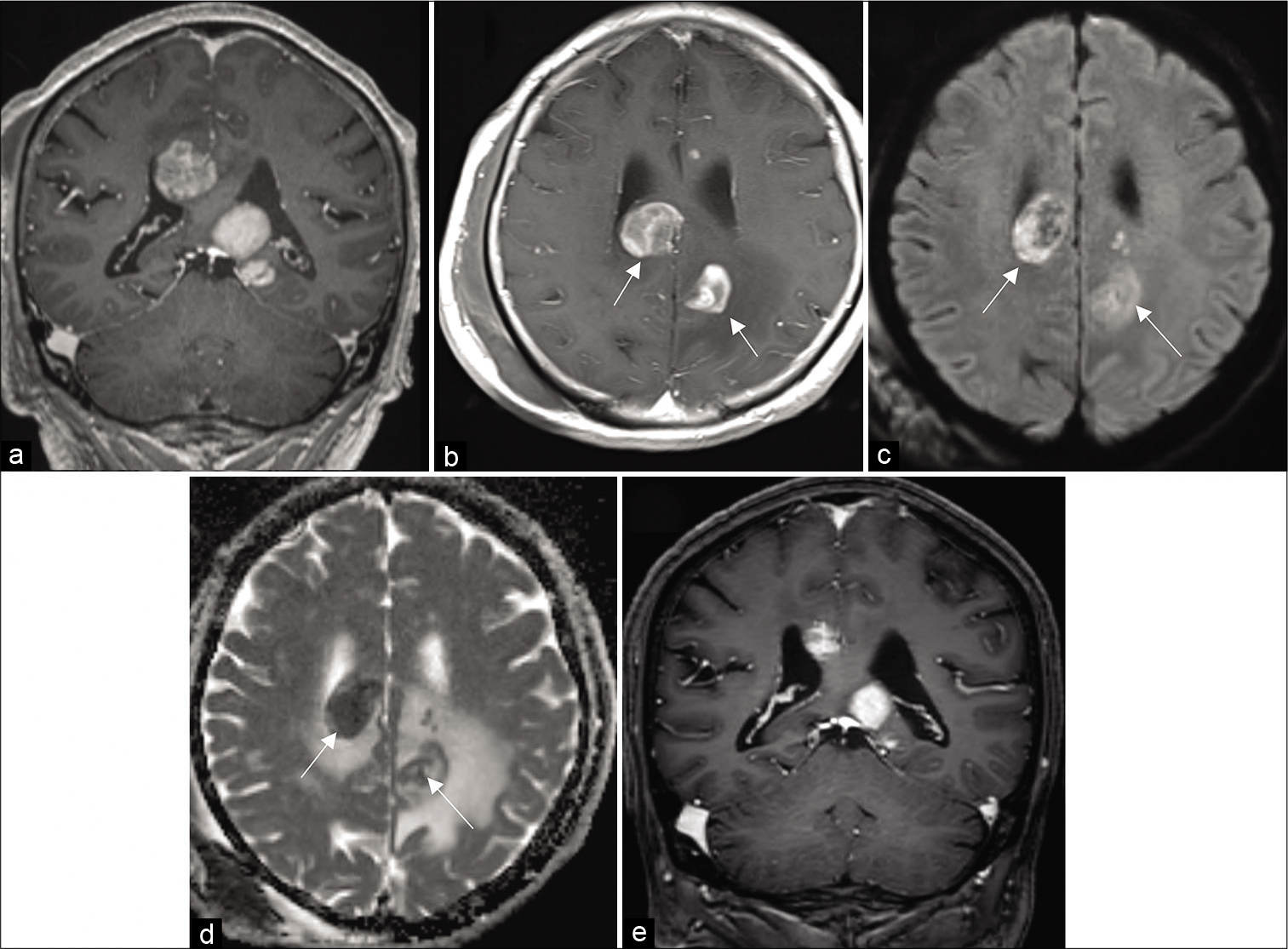
A 78-year-old female initially presented to a local hospital with a history of fall, sustaining a contusion in the right temporoparietal region. Her neurological examination revealed weakness of the left lower limb and cognitive dysfunction. The head computed tomography (CT) scan showed minimal bilateral intraventricular and right occipital subarachnoid hemorrhage, as well as incidental hyperdense lesions in the corpus callosum, cingulate gyrus, left frontal, and right periventricular regions, for which she was referred to our center. Post-contrast T1-weighted magnetic resonance (MR) imaging revealed that all the hyperdense lesions identified on CT were enhanced, in addition to newly identified lesions in the left occipital lobe and the midbrain, the latter of which was compressing the sylvian aqueduct. The lesion around the corpus callosum showed homogeneous enhancement [
Figure 1:
MRI scan. (a) Multiple enhancing corpus callosum lesions. (b, arrow) Partial open-ring pattern in the left occipital lobe and right periventricular lesion. (c, arrow) High signal on diffusion-weighted imaging. (d, arrow) Low values on ADC map. (e) Spontaneous regression five days after biopsy.
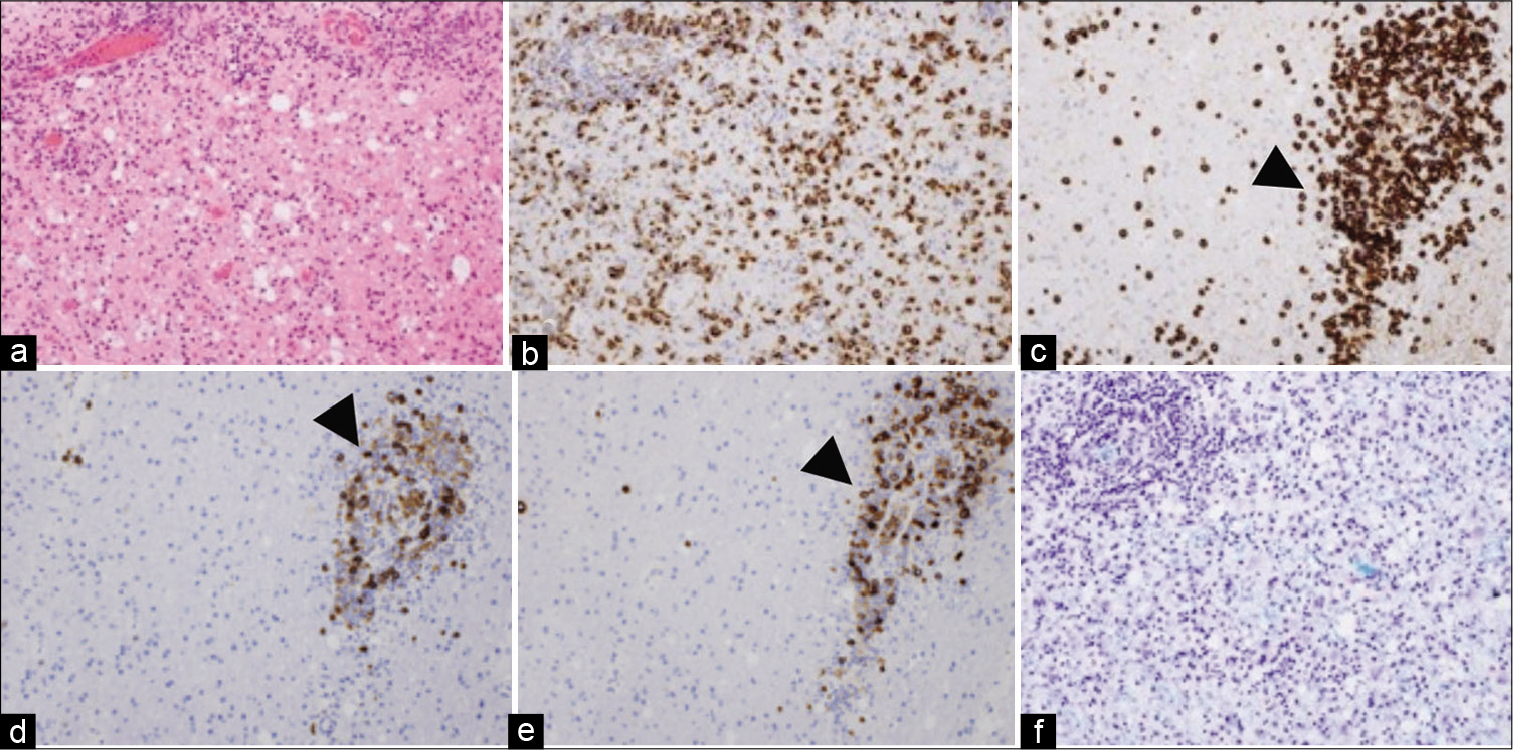
The histopathological report from this biopsy described perivascular and parenchymal lymphocytic infiltration with the presence of large diffuse astrocytes as well as foam cells, and evidence of astrogliosis [
Figure 2:
First biopsy (a) H and E perivascular and parenchymal infiltration, (b) CD68-positive histiocytes, (c, arrowhead) CD8-positive reactive T-lymphocytes, (d, arrowhead). A small number of CD20- and (e, arrowhead) CD79a-positive B-lymphocytes. (f) Demyelinating lesions with Klüver-Barrera staining.
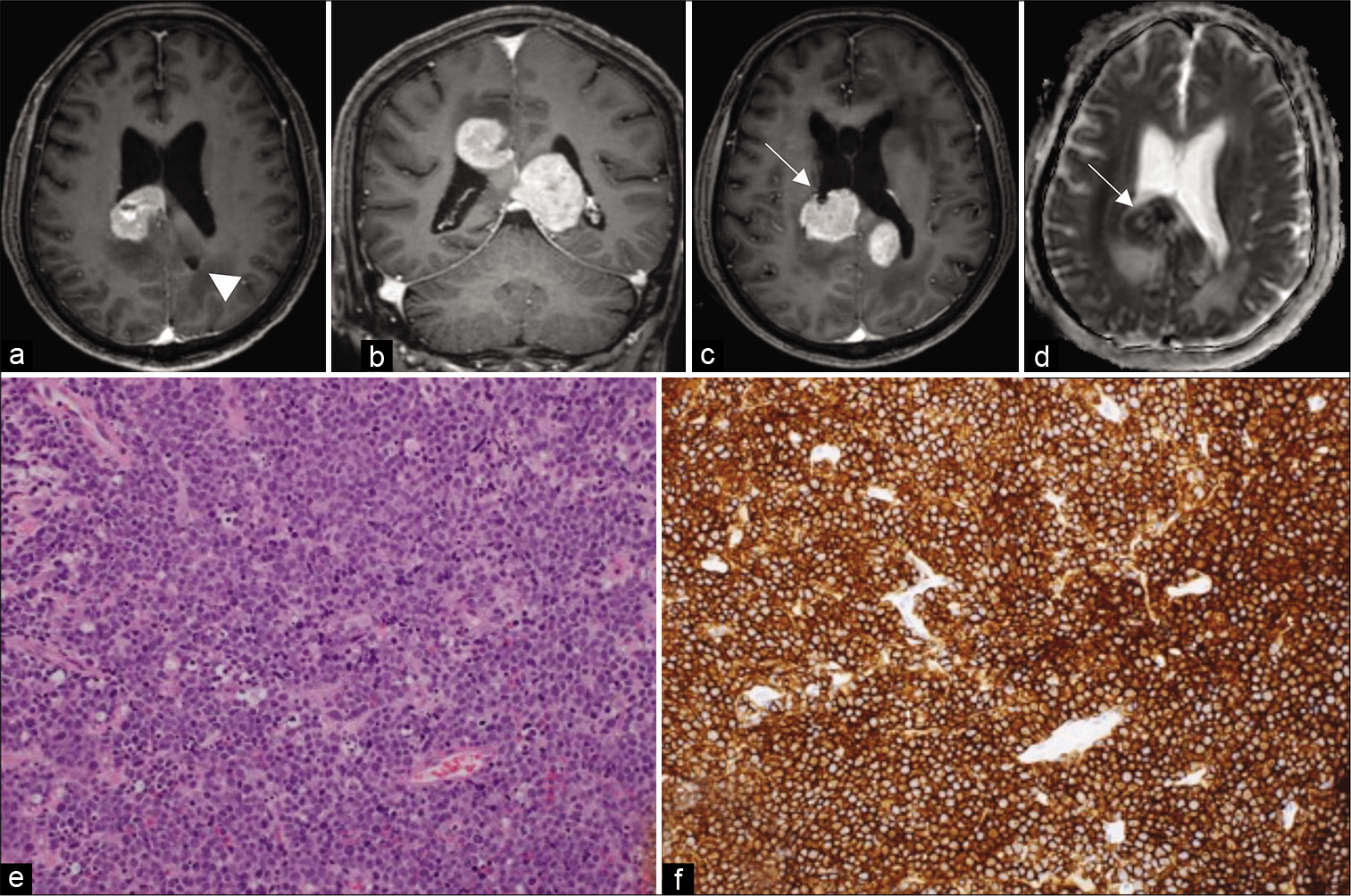
She remained relatively stable for a period of almost 3 months after the initial biopsy but was subsequently re-admitted with progressive disorientation. Post-contrast T1-weighted MR imaging showed gliosis and complete resolution of the previously biopsied lesion in the left occipital lobe [
Figure 3:
MRI three-months after biopsy. (a, arrowhead) Gliotic lesion from the first biopsy. (b) Progressive lesions around the corpus callosum. (c, arrow) Second biopsy site. (d, arrow) Low values on ADC map. (e) H and E staining showing atypical lymphoid cells. (f) CD20-positive B immunophenotype tumour cells.
DISCUSSION
Intracerebral demyelinating lesions that appear before the true diagnosis of PCNSL are rare, when detected initially in non-neoplastic lesions they are described as a “sentinel lesion”.[
Several hypotheses have been proposed regarding the pathophysiology of sentinel demyelinating lesions. Kuhlmann et al. noted anti-myelin oligodendrocyte glycoprotein antibody in the serum of PCNSL patients before sentinel demyelination, and explained these antibodies may have been produced by a monoclonal population of transformed B cells, resulting in autoimmune demyelination.[
Histologically, the sentinel lesion is mostly composed of either perivascular or parenchymal infiltration of inflammatory cells and is frequently accompanied by demyelination. Usually, CD3-positive T lymphocytes predominantly possess perivascular cuffing, and in a few cases, infiltration of T lymphocytes is limited to the parenchymal cells alone. Our patient was unique due to the presence of both perivascular and parenchymal lymphocytes. A similar finding was also observed by Husseini et al., but our patient is particularly unique due to the presence of complete demyelination on KB staining. To the best of our knowledge, none of the cases of complete demyelinating sentinel lesions reported to date describe the presence of perivascular and parenchymal infiltration of inflammatory cells at the same time. The reason behind this distinct histological picture is unknown. The unique feature of spontaneous regression in an immunocompetent patient without steroid therapy, and with predominant T cells, advocates the potential presence of a suppressive cell-mediated anti-tumor response.[
CONCLUSION
The sentinel lesion of PCNSL in immunocompetent patients expresses a variable histological pattern of inflammatory cells. The usual perivascular infiltrative pattern is commoner than parenchymal infiltration, and the mixed-pattern involving both perivascular and parenchymal infiltration is exceptionally rare. This case report describes a unique histological finding and describes the recognized variations in sentinel lesion histopathology. Moreover, distinguishing tumefactive MS for sentinel lesion of PCNSL is difficult on radiological study alone. Hence, the accurate diagnosis and treatment of PCNSL require repeated biopsies for histopathological examinations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the technical research assistant Miss Tomoko Takajo from the Department of Neurosurgery, Kagoshima University, for preparing the pathological data.
References
1. Alderson L, Fetell MR, Sisti M, Hochberg F, Cohen M, Louis DN. Sentinel lesions of primary CNS lymphoma. J Neurol Neurosurg Psychiatry. 1996. 60: 102-5
2. Chiavazza C, Pellerino A, Ferrio F, Cistaro A, Soffietti R, Rudà R. Primary CNS lymphomas: Challenges in diagnosis and monitoring. Biomed Res Int. 2018. 2018: 3606970
3. Husseini L, Saleh A, Reifenberger G, Hartung HP, Kieseier BC. Inflammatory demyelinating brain lesions heralding primary CNS lymphoma. Can J Neurol Sci. 2012. 39: 6-10
4. Javier R, Shaikh N, Lesniak MS, Sonabend A, Stupp R, Behdad A. B cell-rich non-neoplastic sentinel lesion preceding primary central nervous system lymphoma. Diagn Pathol. 2018. 13: 37
5. Kalus S, Di Muzio B, Gaillard F. Demyelination preceding a diagnosis of central nervous system lymphoma. J Clin Neurosci. 2016. 24: 146-8
6. Kuhlmann T, Schröter A, Dechent P, Weber F, Rustenbeck HH, Füzesi L. Diagnosis of a multifocal B cell lymphoma with preceding demyelinating central nervous system lesions by single voxel proton MR spectroscopy. J Neurol Neurosurg Psychiatry. 2001. 70: 259-62
7. Kuroda Y, Kawasaki T, Haraoka S, Fujiyama F, Kakigi R, Abe M. Autopsy report of primary CNS B-cell lymphoma indistinguishable from multiple sclerosis: Diagnosis with the immunoglobulin gene rearrangements analysis. J Neurol Sci. 1992. 111: 173-9
8. Kvarta MD, Sharma D, Castellani RJ, Morales RE, Reich SG, Kimball AS. Demyelination as a harbinger of lymphoma: A case report and review of primary central nervous system lymphoma preceded by multifocal sentinel demyelination. BMC Neurol. 2016. 16: 72
9. Louis DN, Wiestler OD, Ohgaki H.editors. WHO Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2016. p.
10. Lu JQ, O’Kelly C, Girgis S, Emery D, Power C, Blevins G. Neuroinflammation preceding and accompanying primary central nervous system lymphoma: Case study and literature review. World Neurosurg. 2016. 88: 692.e1-8
11. Ng S, Butzkueven H, Kalnins R, Rowe C. Prolonged interval between sentinel pseudotumoral demyelination and development of primary CNS lymphoma. J Clin Neurosci. 2007. 14: 1126-9
12. Ohe Y, Hayashi T, Mishima K, Nishikawa R, Sasaki A, Matsuda H. Central nervous system lymphoma initially diagnosed as tumefactive multiple sclerosis after brain biopsy. Intern Med. 2013. 52: 483-8
13. Paydas S. Primary central nervous system lymphoma: Essential points in diagnosis and management. Med Oncol. 2017. 34: 61
14. Schlegel U. Primary CNS lymphoma. Ther Adv Neurol Disord. 2009. 2: 93-104
15. Yamamoto J, Shimajiri S, Nakano Y, Nishizawa S. Primary central nervous system lymphoma with preceding spontaneous pseudotumoral demyelination in an immunocompetent adult patient: A case report and literature review. Oncol Lett. 2014. 7: 1835-8