- Department of Neurosurgery, Shanghai Children's Medical Center Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, People's Republic of China
- Department Neurosurgery, UCLA Center for World Health at David Geffen School of Medicine, Tiverton Drive, Los Angeles, CA 90024, USA
Correspondence Address:
Nan Bao
Department Neurosurgery, UCLA Center for World Health at David Geffen School of Medicine, Tiverton Drive, Los Angeles, CA 90024, USA
DOI:10.4103/2152-7806.161410
Copyright: © 2015 Bao N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.How to cite this article: Bao N, Lazareff J. How I Do It: Management of spina bifida in a hospital in The People's Republic of China. Surg Neurol Int 23-Jul-2015;6:
How to cite this URL: Bao N, Lazareff J. How I Do It: Management of spina bifida in a hospital in The People's Republic of China. Surg Neurol Int 23-Jul-2015;6:. Available from: http://surgicalneurologyint.com/surgicalint_articles/how-i-do-it-management-of-spina-bifida-in-a-hospital-in-the-peoples-republic-of-china/
Abstract
We present our personal experience on patients with Spina Bifida. It is the result of having treated 1600 children for 12 years at Shanghai Children's Medical Center. We classify the cases on Spina Bifida Manifesta (myelomeningocele, myelocele, lypomyelomeningocele) or Spina Bifida Oculta (lipoma, dermal sinus and thickened filum terminale). For the former, we recommend surgery within 24–48 h after birth. For the latter we recommend preventive surgery months after birth. We acknowledge that the diameter of the spinal canal is a problem for large remnant lesions. In cases of myelomeningocele, we prefer to place the shunt and close the defect in the same procedure, it reduces the risks inherent to exposure to anesthesia, reduces hospital stay, and related costs. If there is a suspicious of infection, we do not place the shunt on the same procedure. The personal description of the preferred techniques for closure of the different defects is described.
Keywords: Myelomeningocele, spina bifida, surgical technique, spinal lipoma, tethered cord
CLASSIFICATION
There are several types of neural tube defects and each type can be divided into various subtypes. According to our experience in the treatment of nearly 1600 patients with different types of neural tube defects at the Neurosurgery Department of Shanghai Children's Medical Center over the past 12 years, we divided common neural tube defects into the following types:
Spina bifida manifesta
This type can be further divided into the following subtypes according to the pathological morphology:
Myelomeningocele
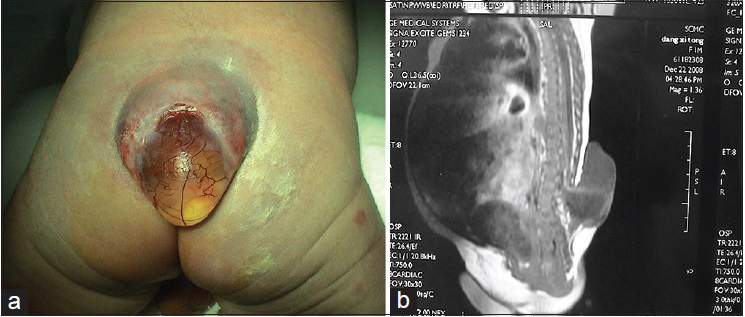
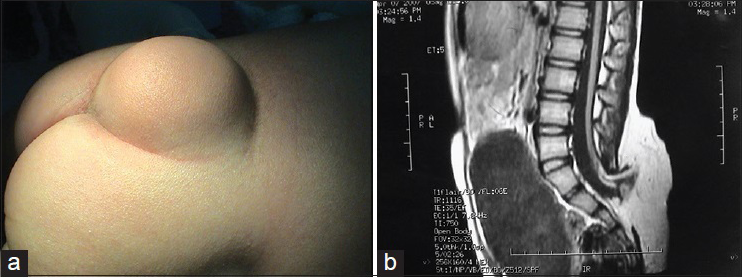
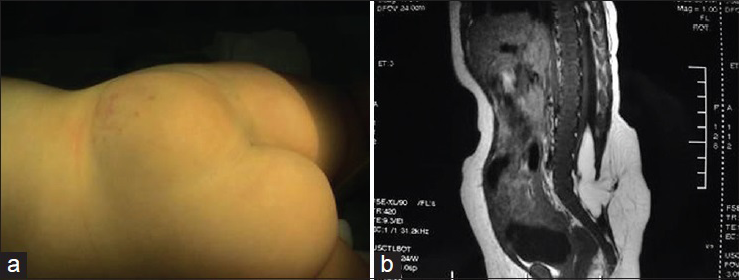
Patients with this subtype of neural tube defect have a mass on their back. The surface of the mass is a thin cyst wall and in some cases there is no skin [
Neurological damage is very serious in patients with myelomeningocele. Most patients with type I myelomeningocele have bladder-sphincter dysfunction and most patients with type II myelomeningocele have lower extremity dysfunction, foot deformities, and even bladder-sphincter dysfunction and spinal deformities, etc., The incidence of Chiari malformation and hydrocephalus is as high as 99%, and these conditions are followed in incidence by syringomyelia, diastematomyelia, arachnoid cyst, etc.
Myelocele
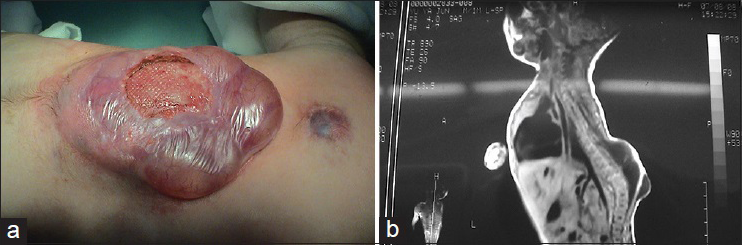
Patients with this subtype of neural tube defect have a mass on their back. There is purple granulation at the center of the mass, which is surrounded by a thin cyst wall. The purple granulation is actually the Ω-shaped herniated spinal cord, which is directly exposed outside of the skin [
This subtype is commonly seen in the lumosacral, lumbar, and thoracolumbar segments is accompanied by the most serious neurological damage, and is associated with lower extremity dysfunction, foot deformities, bladder-sphincter dysfunction, spinal deformities, Chiari malformation, hydrocephalus, etc.
Lipomyelomeningocele
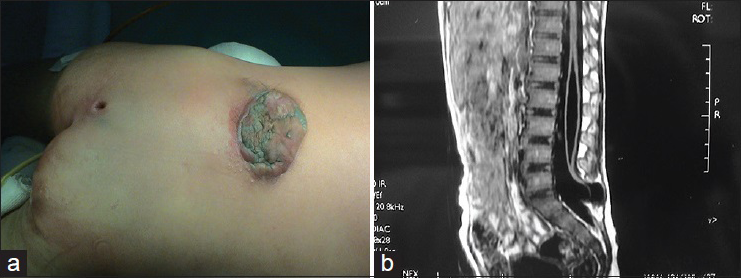
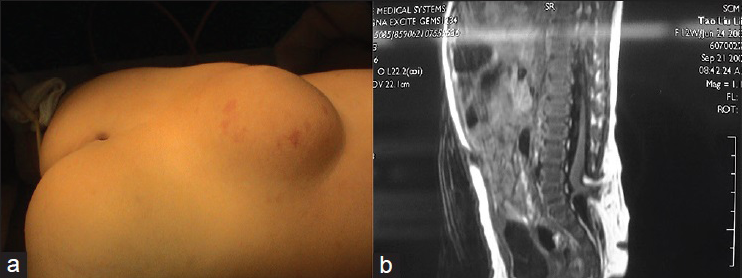
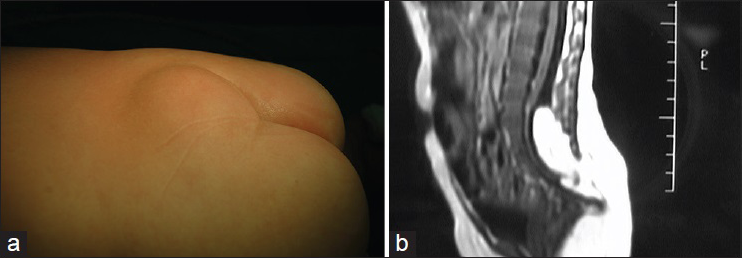
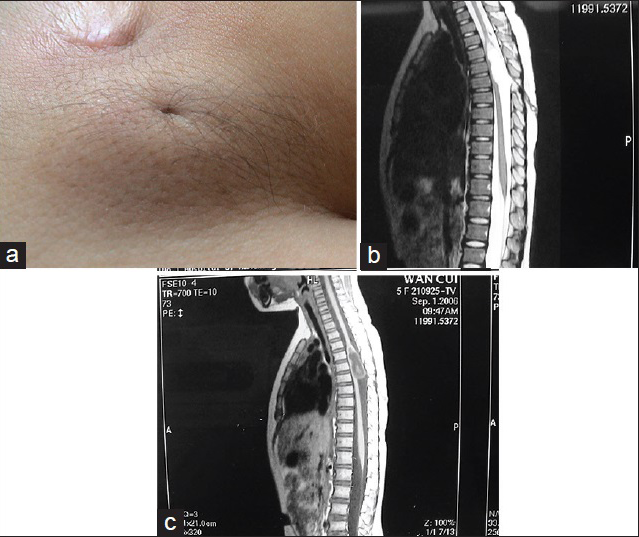
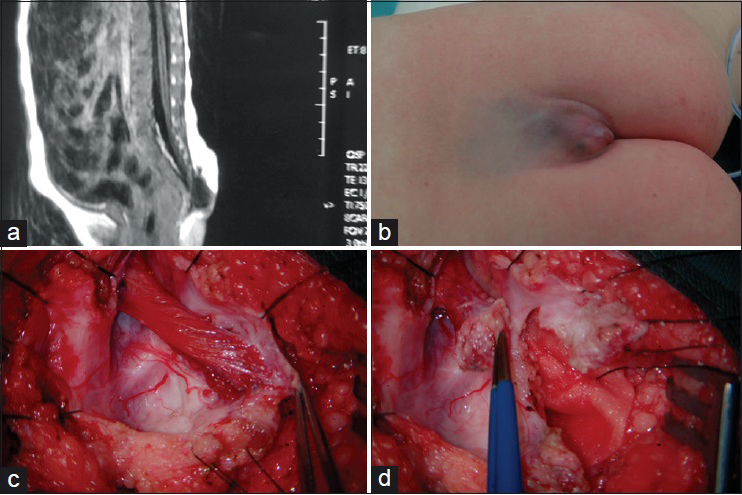
In this subtype the enlarged spinal cord protrudes dorsally via the defect in the spinal canal to form a mass that protrudes over the skin surface. The skin on the surface of the mass is intact. The mass contains subcutaneous fat tissue, cerebrospinal fluid (CSF), and spinal cord. The subcutaneous fat grows together with the herniated spinal cord and dura mater to form the cyst roof. Similar to myelomeningocele, this subtype can be divided into two subtypes according to the different morphologies of the herniated spinal cord. Type I is a herniation of the end of the spinal cord and is commonly seen in the lumbosacral and sacral segments [
Lipomyelomeningocele is a mass covered with normal skin, and there may be abnormal pigmentation or skin depression on the mass surface. Patients with lipomyelomeningocele may have varying degrees of lower extremity paralysis, foot deformities, gait abnormality, and bladder-sphincter dysfunction. Severe cases are often associated with Chiari malformation, hydrocephalus, cerebral dysplasia, hydromyelia, diastematomyelia, etc. Late symptoms include scoliosis, hydronephrosis, etc.
Simple meningocele
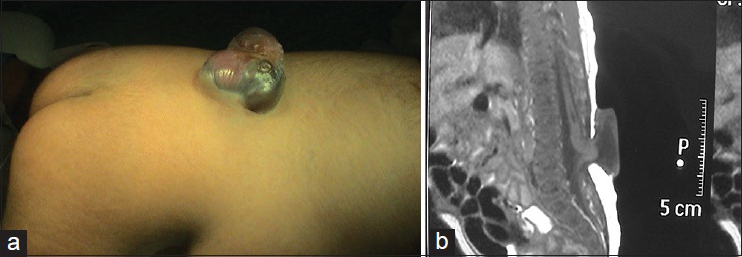
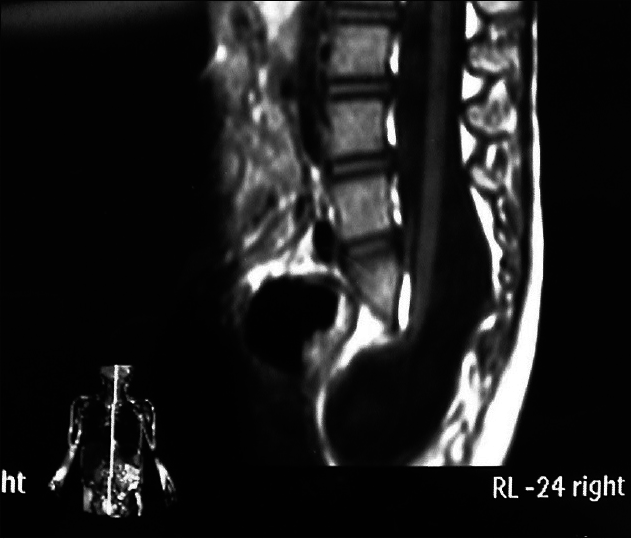
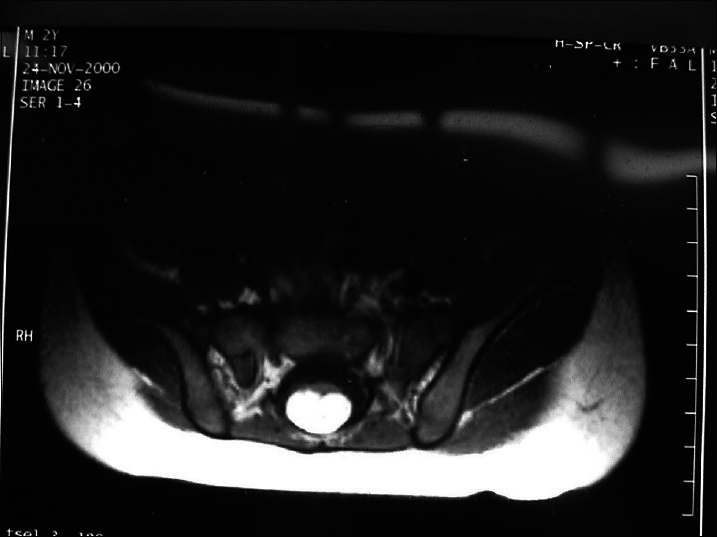
This subtype is characterized by bulging of the dura mater from the bone defect, and the cyst contains only CSF without spinal cord or cauda equina. If the cyst protrudes dorsally from the spinal canal, it is called a posterior simple meningocele [
Occult spinal bifida
Skin in the affected area often has characteristic features including pigmentation, capillary hemangioma, skin depression, local hirsutism, small skin tags, etc. No significant symptoms can be observed in infants, and tethered spinal cord syndrome appears during the gradual development of the child after abnormal traction of the spinal cord occurs. It was reported that many patients have symptoms only after they grow up. Neural injury is mostly caused by the compression, traction, or increased tension of the spinal cord. If there is no surgical treatment, neural injury will become further aggravated and irreversible. Therefore, early diagnosis is very important for performing surgical intervention as soon as possible.
Occult spinal bifida is further divided into the following subtypes:
Spinal cord lipoma
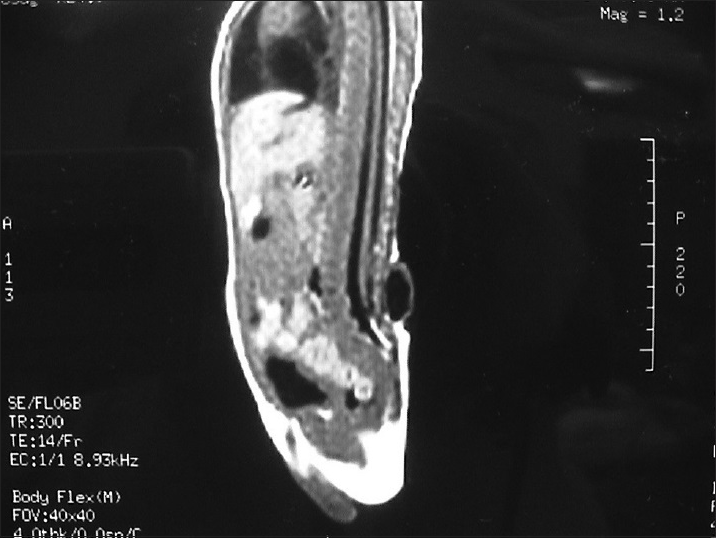
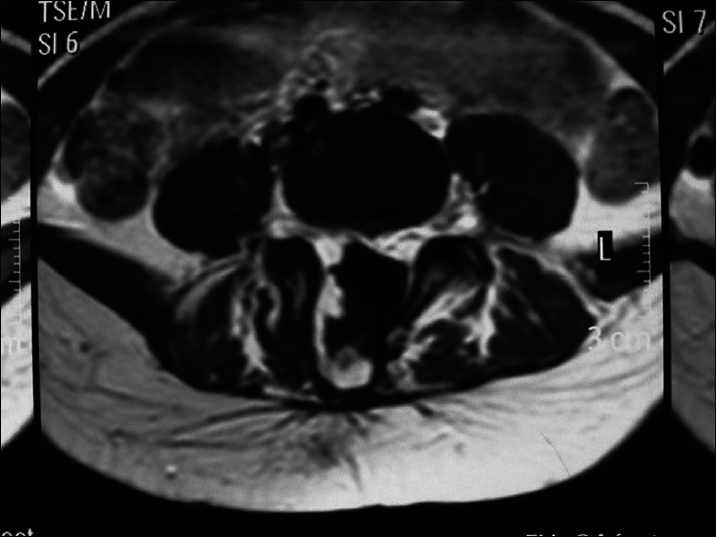
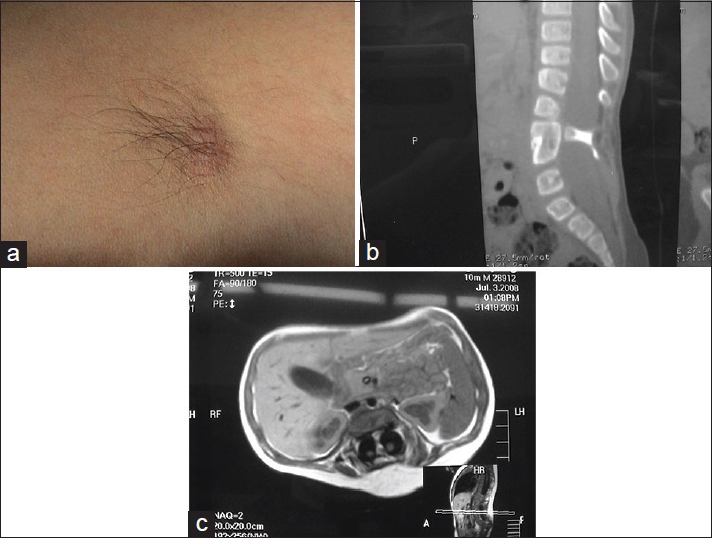
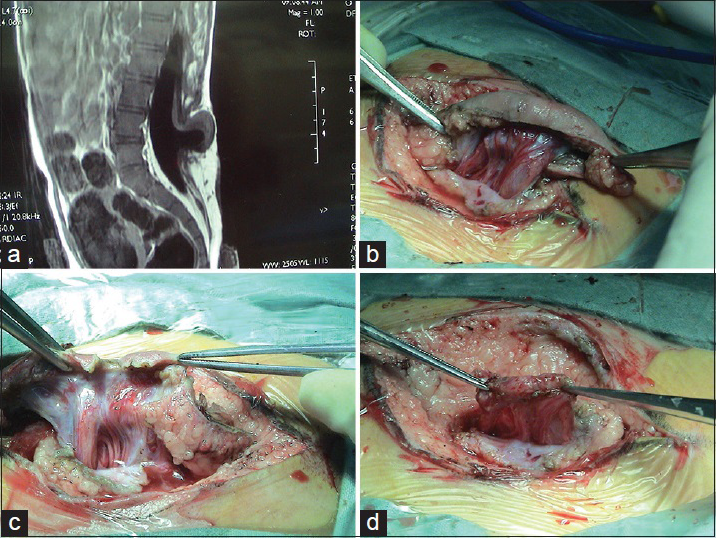
A large amount of subcutaneous fat moves into the spinal canal via the spinal defect, and the dorsal dura mater is completely invaded by the subcutaneous lipoma and loses its normal structure. The lipoma enters the subdural space and grows with the lower located spinal cord. If the lipoma grows into the superficial part of the dorsal spinal cord, it is called a dorsal spinal cord lipoma [
A spinal cord lipoma is seen as a subcutaneous lipoma in the back with a capillary nevus or skin depression. Sometimes, it is only evident as a small skin tag. Affected patients may have varying degrees of bladder-sphincter dysfunction, lower extremity paralysis, foot deformities, and gait abnormality.
Spinal cord lipomas are commonly seen in the lumbosacral and sacrococcygeal segments, and are called lumbosacral spinal cord lipoma [
Unlike a lumbosacral spinal cord lipoma, a sacrococcygeal spinal cord lipoma is located in the sacral canal. The subcutaneous lipoma in the sacrococcygeal region invades the dura mater via the sacral canal defect and grows together with the lower located spinal cord. Instead of terminating in the middle of the lumbosacral dural sac, the conus medullaris terminates in the distal end of the dural sac. Therefore, the cauda equina does not extend longitudinally from the end of the conus medullaris in accordance with normal anatomy, but extends obliquely downward from the ventral side of the conus medullaris. The lipoma is located in the superficial layer of the conus medullaris and grows into the conus medullaris. Differences in surgical procedures will be described in detail in the part on treatment.
Back dermal sinus
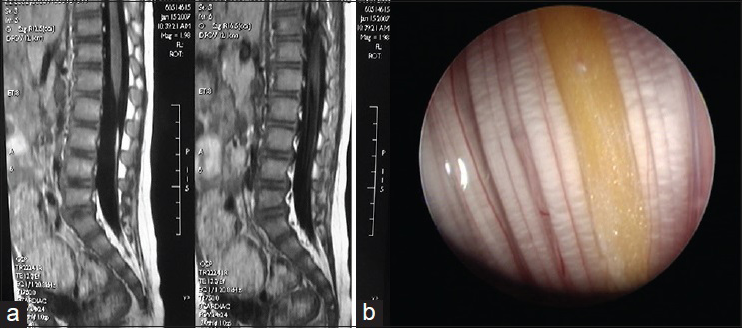
This subtype develops on the dorsal side of the cerebrospinal axis, at any site between the occiput and the sacrococcygeal region, most commonly in the lumbosacral region. The sinus may terminate outside or inside the dura mater. At the termination of the sinus is often a dermal cyst, which is located at the end of the spinal canal or grows into the spinal cord and causes tethered spinal cord [
It is seen as pinprick-like holes on the skin with peripheral abnormal hairs, pigmentation, or capillary hemangioma-like changes. Surgery should be performed as early as possible to prevent serious results such as secondary cyst infection, cerebrospinal meningitis, etc.
Diastematomyelia
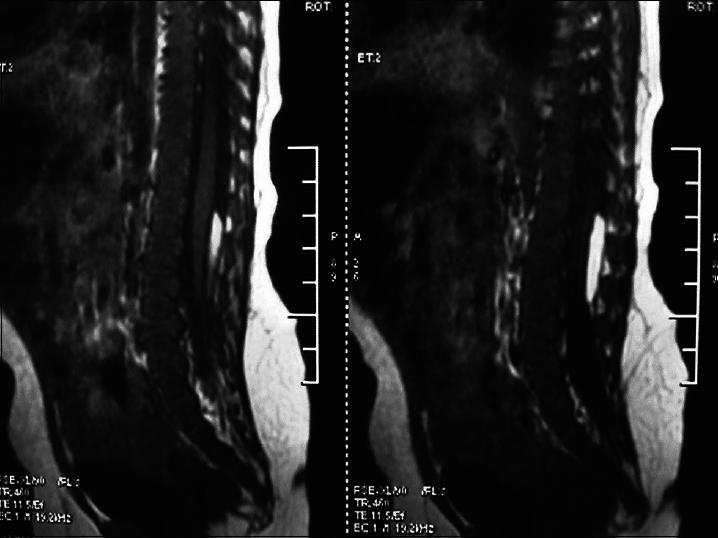
This subtype can be further divided into two subtypes according to the presence or absence of clinical symptoms: Type I (presence of symptoms): There are two dural sacs and two spinal cords, that is, the spinal cord is divided into two parts. Each has its own dura mater and arachnoid mater and there is fibrous tissue, cartilage, or bone crest between the two parts, which causes tethered spinal cord [
It is commonly seen in the thoracic and lumbar segments, and is seen as an abnormal hair clump in the center of the back. Ninety percent of patients with diastematomyelia have associated scoliosis.
Thickened filum terminale syndrome
When the terminal filament is invaded by fat and fibrous tissues, it degenerates and becomes thickened, consequently pulling down the spinal cord and causing neural symptoms. The neural symptoms caused by the tethered terminal filament are often relatively mild, and patients only have pain, enuresis, urinary urgency, fecal leakage, pes cavus, slight foot varus or valgus, etc. Location of the conus medullaris may be normal or lower [
Intradural lipoma
There is a localized fat accumulation in the subdural space without connection to the subcutaneous fat tissue in the back. The lipoma is often adhered to the dura mater on one side and located on the surface of the spinal cord on the other side. It also can grow into the spinal cord and cause tethered spinal cord [
Treatment
Aims: The goal of treatment is to improve neurological function and prevent further neural degeneration.
Ideal age and indication for surgery: Myelocele and myelomeningocele without skin covering may result in continuous CSF leakage due to the thin cyst wall. To reduce the risks of CSF leakage and infection, and subsequent continuous degeneration of neurological function, we often perform surgery to close the defect within 24–48 h after birth. For skin-covered neural tube defects, such as lipomyelomeningocele, elective surgery should be performed after considering the patient's age, body weight, general condition, and tolerance of the delicate spinal cord to outside intervention. Because the tethering and compression caused by the disease during the growth of the spinal cord may lead to further dysfunction, we perform surgery for patients with lipomyelomeningocele and spinal cord lipomas within 2–3 months after birth. However, the spinal canal is thinner in 2- to 3-month-old infants while the protruded spinal cord is abnormally thick, which makes complete reduction of the spinal cord difficult, produces extensive spinal cord adhesion, and increases the risk of subsequent tethered spinal cord. Therefore, the most suitable age for surgery in these children should be further investigated.
In patients with occult spinal bifida the disease is often detected a few years after birth based on manifestations including thickened filum terminal syndrome, diastematomyelia, and back dermal sinus. Surgery is often performed late in these patients.
Myelocele and myelomeningocele are associated with a high incidence of hydrocephalus. With regard to hydrocephalus requiring shunt surgery, there are differing opinions about whether shunt surgery should be performed together with surgeries for the original diseases or whether surgery should be carried out in two stages. We agree with those who advocate one-stage surgery because it allows the patients to undergo surgery and anesthesia only once, shortens the length of hospital stay, and reduces the medical cost. Because surgery for hydrocephalus is relatively less associated with problems, and CSF loss occurs during myelomeningocele repair, which may decrease the volume of the cerebral ventricle and make ventricular puncture become difficult, we perform shunt surgery after placing the patient in the supine position and then carry out myelomeningocele repair after turning the patient over to the prone position. To reduce the rate of complications including shunt occlusion and infection, we carry out strict decontamination of the surgical site, use very strict aseptic technique during surgery, minimize intraoperative loss of CSF, prevent cephalic spread of bloody CSF, reduce the time of wound exposure, etc., Of course, for patients with definite or potential infection of the central nervous system, myelomeningocele repair is done first and second-stage hydrocephalus shunt surgery is performed after the infection of the central nervous system is controlled.
Surgical principle: The spinal cord should be separated from the adhered lesion and the lesion removed to relieve spinal cord compression and tethering.
Surgical technique: Because the tumor surface is not covered by skin in patients with myelocele and myelomeningocele, the site of defect should be wrapped with saline-moistened gauze immediately after the patient is brought into the NICU to avoid drying and direct injury of the neural substrates due to exposure. The patient should be placed in a supine or lateral recumbent position and administered antibiotics intravenously.
Various measures should be used to prevent possible intraoperative hypothermia. For example, we increase the temperature of the operating room and put a heating pad underneath the child, avoid wrapping the abdomen and chest using wet towels during operation, and perform surgery as soon as possible according to the plan. The whole procedure should be carried out under microscope. The patient is placed in a prone position and a soft pad is placed underneath the lower abdomen to elevate the lower abdomen and buttock to the level of the head to maximally reduce CSF leakage during the procedure.
At the beginning of the surgery, an incision is made along the margin of exposed neural placode and the placode is separated from the peripheral tissue to get it back into the spinal canal. All the contents are trimmed including skin and granulation tissue on the placode. A neural tube resembling the spinal cord is then reconstructed, and the pia mater and arachnoid mater are drawn close to the midline from the bilateral margins of the placode and sutured. All the neural tissues are carefully protected and special attention is paid to electrocoagulation in order to avoid thermal burn injury to the placode, which may have residual function.
The dura mater is then dissected. One side of the dura mater is adjacent to the margin of the skin defect and is completely isolated from the skin. The dura mater is closed using 5-0 absorbable suture line without compressing the spinal cord.
Finally, the skin is sutured. If a patient has a large myelomeningocele, the large-area skin defect cannot be repaired by simple closure. Therefore, Z-shaped skin flaps are used or large-scale subcutaneous dissection is performed as far as both sides of the back to ensure a tension-free skin closure. Blunt finger dissection is carried out to avoid damaging large blood vessels. This kind of dissection can be performed quickly, which not only reduces bleeding but also ensures blood supply to the skin flap. Use of Z-shaped skin flaps or performing large-scale subcutaneous dissection guarantees direct skin suture for all patients.
In patients with a lipomyelomeningocele, after the cyst is separated, most of the time, half of the laminae of the vertebrae superior and inferior to the spina bifida is removed to sufficiently expose the base of the cyst. The normal dura mater is cut open to identify the relationship between the herniated spinal cord and myelomeningocele to avoid damaging the spinal cord when separating the cyst wall. An incision is made at the side of the apex of the cyst containing protruded spinal cord, the nerve within the cyst is carefully protected, and annular resection of the remaining parts is done under direct vision. Complete dissection is performed to release the spinal cord and neural fibers adhered to the cyst wall. Because a subcutaneous lipoma invades the spinal cord in cases with lipomyelomeningocele, the fat outside the spinal cord should be removed using a scissor and the fat inside the spinal cord should be removed using a micro scissor or an ultrasonic aspirator as much as possible to expose the layer of neural placode. Finally, interrupted suturing is performed for the split spinal cord, which is placed into the spinal canal. The redundant bulging dura mater is trimmed, and expanded suturing to the dural sac is done to prevent neural tissue compression and adhesion.
Surgical procedures for the two subtypes of myelomeningocele or lipomyelomeningocele are similar. For type I, the only requirement is to dissect and cut off the herniated distal end of the spinal cord from the bulging dura mater [
For simple meningocele, a fusiform incision should be made around the mass and dissection should be performed from the outside of the cyst wall to the neck of the cyst. The cyst wall should be cut open at the apex to explore the presence of neural tissue. According to the preoperative MRI findings, the bottom of the cyst cavity should be slightly expanded to explore the spinal canal, and find out whether there is fat degeneration of filum terminale, fibrous band tethering of the spinal cord, and adhesion between the dura mater and the spinal cord or the cauda equina. Corresponding tethering should be released. The redundant cyst wall should be trimmed and the dura mater sutured at the base. The par spinal muscle and fascia should be dissected around the laminar defect and the spinal defect covered using the reinforced suture technique.
Because there is no definite boundary between an intramural lipoma and normal spinal cord, complete resection of the lipoma is impossible. The aim of surgery is to dissect adhesion between the lipoma and the dura mater, reduce the volume of the lipoma, and release spinal cord tethering and compression to allow the reconstructed spinal cord to be suspended in the subarachnoid space satisfactorily.
The surgical technique for a spinal cord lipoma involves cutting open the dura mater from the cephalic normal site until the lipoma is completely exposed. The tumor membrane should be cut open and the lipoma should be gradually removed outside the spinal cord with a micro scissor. After most lipoma lesions outside the spinal cord are removed and the spinal cord is decompressed, the spinal cord should be slowly lifted from the ventral side of the spinal canal. At this point, the boundary of spinal cord is not yet isolated and the lipoma close to the spinal cord surface should not be removed in a hurry to avoid damaging the spinal cord below it. The spinal cord should be gently retracted to one side to expose the dura mater on the lateral side. The spinal cord should be dissected and cut off from both sides of the dura mater using a micro scissor in a cephalic to caudal direction to release the tethering and expose the spinal cord boundary. Then the fat and fibrous tissue should be further removed from the spinal cord surface safely, effectively, and maximally using the ultrasonic aspirator or CO2 laser knife until the layer of the neural plate is exposed. The small amount of fat tissue within the spinal cord should not be forcefully removed in order to protect spinal cord function.
Surgery for sacrococcygeal spinal cord lipoma is more difficult than surgery for lumbosacral spinal cord lipoma. In patients with lumbosacral spinal cord lipoma, the conus medullaris is located in the middle segment of the lumbosacral dura mater and the boundary is easily observed. As long as the normal dura mater is dissected at the caudal end of the lipoma and cut open cephalically, the conus medullaris can be identified and isolated from the dura mater. However, in patients with sacrococcygeal spinal cord lipoma, the conus medullaris is located in the distal end of the dural sac. The lipoma on the surface of the conus medullaris not only grows together with the conus medullaris, but also grows outside the sacral canal and connects with the normal fat tissue in the sacrococcygeal area without boundaries. Because the lipoma completely covers the conus medullaris and distal end of the dura mater, it is extremely difficult to identify the boundary between the conus medullaris and distal end of the dura mater during surgery and separate them. Our method is to diminish the fat from the cephalic end, lift the spinal cord from the ventral side after the fat becomes thinned, and then cut off the spinal cord from the dura mater. When the spinal cord boundary is exposed, the fat inside the spinal cord should be further removed in a cephalic to caudal direction, and the lipoma of the conus medullaris and tethering should then be treated. The key point is to accurately identify the boundary between the distal end of the dural sac and the conus medullaris in order to move toward the midline from both sides, dissect the conus medullaris, and release it from the end of the dural sac. If dissection toward the midline is performed too early, it will cut off and damage the conus medullaris, and if the dissection is carried out too late, it may cause disorientation and result in the manipulation being moved outside the caudal thecal sac or even the sacral canal, which not only cannot release the conus medullaris tethering, but also may damage the sacrococcygeal epidural spinal nerve. The fat on the surface of the conus medullaris should be diminished. When the fibrous fat layer is exposed, the dural sac end on the surface of the conus medullaris should be initially identified. The conus medullaris should be gently pulled aside at this point to further identify the distal end of the dural sac inside the sacral canal because the course of the dural sac end slants upward within the sacral canal. After the sacral sac end is reached, dissection should be carried out from both sides to the midline to completely isolate the conus medullaris from the end of the dural sac and entirely release the tethered spinal cord. After the boundary of the conus medullaris is totally exposed, the fibrous fat tissue on the surface of the conus medullaris should be removed safely and effectively and the neural plate reached.
Finally, the opened spinal cord should be repaired with interrupted suture to reduce the risk of postoperative adhesion between the dorsal side of the spinal cord and the suture site in the dura mater, and minimize the possibility of secondary tethering.
The key point of surgery for diastematomyelia is to remove the septum, regardless of bone, fat, or cartilage, because it is the cause of tethering. The bony septum outside the dura mater should be removed as much as possible using a ranger or small awl. In most cases, there are many blood vessels around the septum, which may cause massive bleeding if injured. The dura of the two spinal cords should be cut open. Often there are fibrous adhesions between the spinal cord and the dura mater at the site of the septum, and any such adhesions should be completely separated.
Surgery for thickened filum terminale syndrome should be performed via an incision between L4 and L5 or L5 and S1 spinouts processes. The dura mater and arachnoid mater should be cut open and the filum terminale identified according to midline location, yellow or silver change of filum’ color, disappearance of nodes of Rangier, and fat infiltration. The filum terminale should be separated from the peripheral nerve and slightly rotated to identify the presence of nerve adhesion on the ventral side. After electrocoagulation, 5 mm of filum terminale should be cut off as a specimen for pathological evaluation.
Surgery for a back dermal sinus requires complete removal of the dermal cyst and sinus inside and outside the spinal cord. It is necessary to identify the terminal of the sinus. Though sometimes a back dermal sinus that terminates on the surface of the dura mater is shown in imaging examinations, cutting open the dura mater is still needed for exploration because some tiny dermal cysts within the dura mater are often not shown on MRI.
Postoperative follow-up
Because some changes such as swelling caused by surgery disappears at least 1 month after surgery on MRI images, we often perform MRI 2–3 months after surgery to learn the postoperative status of the spinal cord. Close observation is carried out for patients with myelocele or myelomeningocele to identify the presence or absence of progressive aggravation of hydrocephalus. If the neurological function is stable, we regularly follow-up our patients 1, 3, and 5 years after surgery. We have a complete patient database and every patient is followed up by an experienced doctor. This may help doctors obtain first-hand information, learn the postoperative status of neurological function, find problems, and in time, adjust or improve surgical procedures.
ACKNOWLEDGEMENT
This work has been supported by the UCLA Center for World Health.







Jesus Farinas Yanes
Posted November 16, 2016, 2:52 pm
excellent review on this topic, congratulations.