- Department of Clinical Neurological Sciences, Western University, London, Ontario, Canada
- Department of Medical Imaging, London Health Sciences Centre, Western University, London, Ontario, Canada
Correspondence Address:
Michael D. Staudt
Department of Clinical Neurological Sciences, Western University, London, Ontario, Canada
Department of Medical Imaging, London Health Sciences Centre, Western University, London, Ontario, Canada
DOI:10.4103/sni.sni_388_18
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Alan Chalil, Michael D. Staudt, Stephen P. Lownie. Iatrogenic pseudoaneurysms associated with cerebrospinal fluid diversion procedures. 12-Mar-2019;10:31
How to cite this URL: Alan Chalil, Michael D. Staudt, Stephen P. Lownie. Iatrogenic pseudoaneurysms associated with cerebrospinal fluid diversion procedures. 12-Mar-2019;10:31. Available from: http://surgicalneurologyint.com/surgicalint-articles/9253/
Abstract
Background:Cerebrospinal fluid diversion procedures, including ventriculoperitoneal (VP) shunt and external ventricular drain insertion, are common treatments for hydrocephalus. Common complications include obstruction, infection, and hemorrhage. Pseudoaneurysm formation secondary to catheter insertion is a distinctly rare complication, and usually involves the anterior cerebral artery or branches of the external carotid artery (superficial temporal artery or middle meningeal artery).
Case Description:We present the case of a fusiform pseudoaneurysm in a 36-year-old female, which arose from a branch of the middle cerebral artery following VP shunt insertion. Parenchymal and intraventricular hemorrhage at the catheter insertion site developed 15 days postoperatively. The VP shunt was removed, and the aneurysmal segment was coagulated and occluded. Use of a limited dural opening during ventricular catheter placement may have been a factor in pseudoaneurysm formation.
Conclusions:The literature regarding this rare complication is reviewed. Careful consideration should be given to vascular anatomy when planning shunt insertions, and a cruciate dural opening for cortical visualization and coagulation may help avoid this complication. Prompt identification and management of iatrogenic pseudoaneurysms is essential to avoid re-bleeding and associated hemorrhagic complications.
Keywords: Complications, external ventricular drain, hemorrhage, pseudoaneurysm, traumatic aneurysm, ventriculoperitoneal shunt
INTRODUCTION
Cerebrospinal fluid (CSF) diversion procedures, including external ventricular drainage (EVD) and ventriculoperitoneal shunt (VP) insertion, are quite common in neurosurgery. These procedures are indicated for treatment and monitoring of increased intracranial pressure secondary to hydrocephalus, trauma, intraventricular hemorrhage, subarachnoid hemorrhage, or shunt failure.[
Hemorrhage rates following EVD catheter insertion reportedly average around 10%,[
The literature and management strategies regarding this rare complication are reviewed. The current article describes the illustrative case of an adult female who developed a multicompartmental hemorrhage due to an iatrogenic pseudoaneurysm following a VP shunt insertion. Etiology, investigations, and management are discussed. Prompt diagnosis and treatment are essential in avoiding the high morbidity associated with these rare complications.
CASE DESCRIPTION
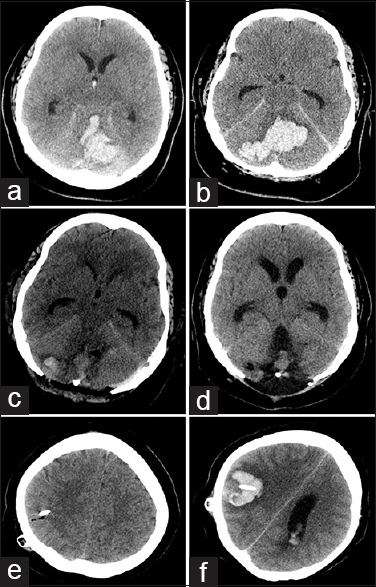
A 36-year-old female with a history of untreated hypertension presented to the emergency department with severe headache and a decreased level of consciousness. Computed tomography (CT) of the head demonstrated a large cerebellar parenchymal hemorrhage [Figure
Figure 1
Axial CT imaging demonstrates initial cerebellar hemorrhage with extension into the third and fourth ventricles and effacement of the basal cisterns (a and b). Immediate postoperative imaging following suboccipital decompression demonstrates good hematoma evacuation (c), with delayed development of communicating hydrocephalus (d). Immediate postoperative imaging following VP shunt insertion does not demonstrate any adverse findings (e), and delayed imaging is consistent with hemorrhage along the shunt tract (f)
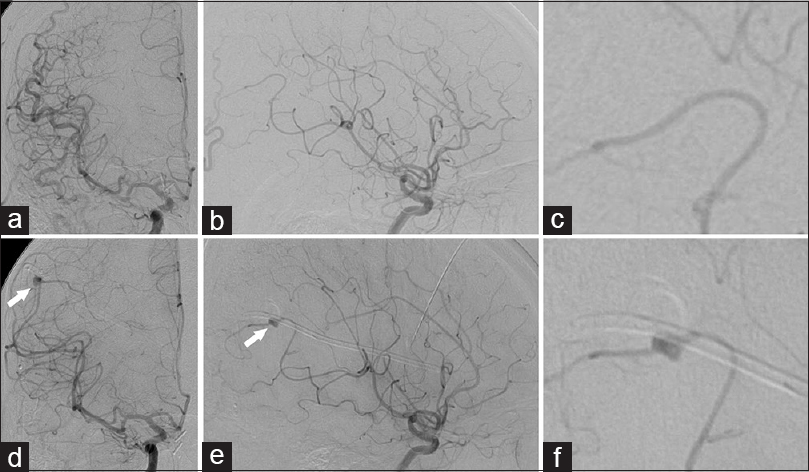
Figure 2
Diagnostic cerebral angiography performed via a right common carotid injection (a: AP; b: lateral; c: lateral zoom) does not demonstrate any aneurysm, arteriovenous malformation, or arteriovenous fistula related to right middle cerebral artery and anterior cerebral artery. Following delayed hemorrhage along shunt tract, right internal carotid injection (d-f) identifies a pseudoaneurysm along the M4 segment of the right middle cerebral artery immediately adjacent to the right parietal ventriculostomy catheter (arrows)
Five weeks following admission, the development of communicating hydrocephalus required the insertion of a VP shunt [Figure
On day 15 following VP shunt insertion, the patient developed a dilated and fixed right pupil. An emergent CT scan demonstrated acute intraparenchymal hemorrhage into the right parietal lobe along the ventriculostomy tract with extensive intraventricular hemorrhage, acute hydrocephalus, and midline shift [Figure
DISCUSSION
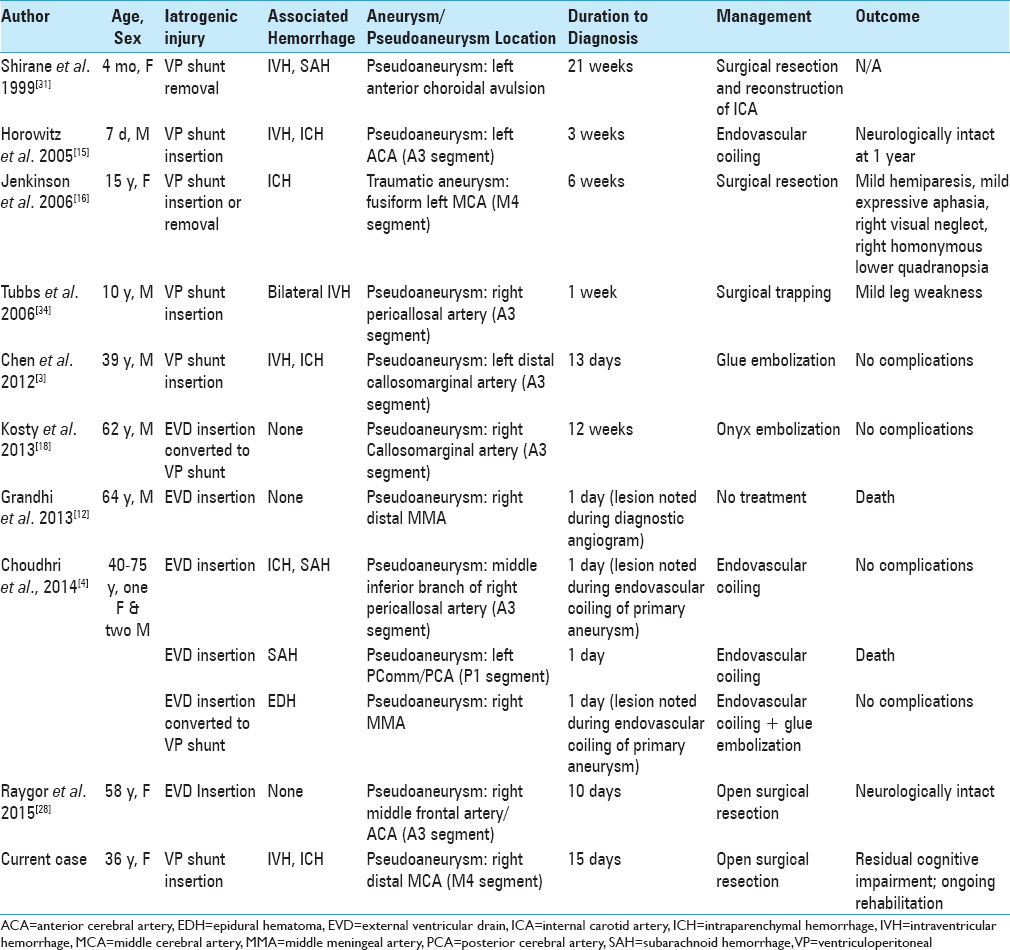
Iatrogenic aneurysms occur mostly in conjunction with injury to a cortical vessel, often presenting as acute blood noted upon the “blind“ insertion of a catheter through a burr hole. In the current case, however, no blood was observed on initial dural puncture or during catheter passage; the thin tip of a Harris forceps was used in conjunction with monopolar cautery to puncture the dura and coagulate the pia. As with other reported cases (summarized in
Etiology and pathophysiology
Traumatic aneurysms are rare, with an incidence of 0.09–0.4% of all intracranial aneurysms;[
Pseudoaneurysms occur after a focal injury to the vessel wall which produces a hematoma in-between the adventitia and muscularis layers at the site of injury. Subsequent resolution of the hematoma forms the outer layer of the newly formed pseudoaneurysm.[
The delay between the initial vessel insult and pseudoaneurysm formation is highly variable, and ranges from 14 to 21 days or more, with some authors reporting delays of many years.[
Pseudoaneurysms in the literature
Pseudoaneurysms secondary to EVD or VP shunt insertion occur in both pediatric and adult patients [
The anterior cerebral artery (ACA) or one of its distal branches was involved in 6 cases of pseudoaneurysm formation following EVD or VP shunt insertion,[
Shirane et al. reported intraventricular and diffuse subarachnoid hemorrhage secondary to a pseudoaneurysm of the proximal internal carotid artery,[
Traumatic aneurysms of the MMA were reported on two occasions in addition to multiple dural arteriovenous fistulas secondary to VP shunt or EVD catheter insertion;[
Traumatic pseudoaneurysms have also been reported secondary to other “blind“ procedures besides EVD and VP shunt insertion. Le et al. reported a traumatic MCA aneurysm from the insertion of an ICP monitor after puncturing the dura with an 18-gauge needle.[
Scalp vessel injuries commonly involved the superficial temporal artery or facial artery, and were associated with tunnelling of the catheter through the scalp after EVD insertion. Occipital artery pseudoaneurysms have also been reported following retrosigmoid craniotomy.[
Principles of management
Pseudoaneurysms carry a relatively high risk of rupture, and require prompt treatment once identified. The duration to identify the lesion ranged between immediate (same day) and up to 21 weeks following VP shunt or EVD insertion. Management consists of standard treatments for intracranial aneurysms, including open surgical clipping or coagulation, and endovascular embolization with coils or glue. Conservative management is not recommended in cases of traumatic aneurysms and pseudoaneurysms due to the high rates of catastrophic bleeds and mortality, which approach 50% in conservatively managed patients compared to 18% in treated patients.[
As with other intracranial aneurysms, several factors contribute to determining the treatment modality, including the location of the aneurysm, morphology, the presence or absence of hemorrhage and associated hematoma, and patient factors including age and comorbidities. Endovascular coiling was the preferred treatment modality in six of the cases, but was found to be of limited value when the pseudoaneurysm involved cortical vessels of small diameter, which limits catheter access.[
CONCLUSION
Iatrogenic pseudoaneurysms are a rare but serious complication of blind procedures, including EVD, VP shunt or ICP monitor insertion, stereotactic brain biopsy, and subdural collection drainage. Attempting proper visualization of the cortex through a generous dural opening is recommended, although not always possible through a small burr hole. Considering the rarity of such complications, it is not necessary to obtain vessel imaging prior to every procedure. However, the suspicion for pseudoaneurysm formation should be high with new intraparenchymal or intraventricular hemorrhage following such procedures, and prompt surgical management is essential.
Declaration of patient consent
Informed consent was obtained for the use of patient history and radiographic images for teaching and research purposes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Benoit BG, Wortzman G. Traumatic cerebral aneurysms. Clinical features and natural history. J Neurol Neurosurg Psychiatry. 1973. 36: 127-38
2. Bratton SL. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007. 24: S37-44
3. Chen Z, Zhang J, Miao H, Niu Y, Feng H, Zhu G. Delayed rupture of iatrogenic cerebral pseudoaneurysms after neurosurgical procedures: Report of two cases. Clin Neurol Neurosurg. 2013. 115: 1552-4
4. Choudhri O, Gupta M, Feroze AH, Heit JJ, Do HM. Endovascular management of external ventricular drain-associated cerebrovascular injuries. Surg Neurol Int. 2014. 5: 167-
5. Ciceri EF, Regna-Gladin C, Erbetta A, Chiapparini L, Nappini S, Savoiardo M. Iatrogenic intracranial pseudoaneurysms: Neuroradiological and therapeutical considerations, including endovascular options. Neurol Sci. 2006. 27: 317-22
6. Dario A, Dorizzi A, Scamoni C, Cerati M, Balcone Grimaldi G. Iatrogenic intracranial aneurysm. Case report and review of the literature. J Neurosurg Sci. 1997. 41: 195-202
7. Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2012. 12: 24-33
8. Fleischer AS, Patton JM, Tindall GT. Cerebral aneurysms of traumatic origin. Surg Neurol. 1975. 4: 233-9
9. Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev. 2012. 35: 485-94
10. Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009. 110: 1021-5
11. Gigante P, Hwang BY, Appelboom G, Kellner CP, Kellner MA, Connolly ES. External ventricular drainage following aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 2010. 24: 625-32
12. Grandhi R, Zwagerman NT, Lee P, Jovin T, Okonkwo DO. Iatrogenic pseudoaneurysm of the middle meningeal artery after external ventricular drain placement. J Neuroimaging. 2015. 25: 140-1
13. Haddad FS, Haddad GF, Taha J. Traumatic intracranial aneurysms caused by missiles: Their presentation and management. Neurosurgery. 1991. 28: 1-7
14. Horiuchi T, Nakagawa F, Miyatake M, Iwashita T, Tanaka Y, Hongo K. Traumatic middle cerebral artery aneurysm: Case report and review of the literature. Neurosurg Rev. 2007. 30: 263-7
15. Horowitz M, Sharts M, Levy E, Albright AL, Pollack I. Endovascular management of ventricular catheter-induced anterior cerebral artery false aneurysm: Technical case report. Neurosurgery. 2005. 57: E374-
16. Jenkinson MD, Basu S, Broome JC, Eldridge PR, Buxton N. Traumatic cerebral aneurysm formation following ventriculoperitoneal shunt insertion. Childs Nerv Syst. 2006. 22: 193-6
17. Karadag A, Kinali B, Ugur O, Oran I, Middlebrooks EH, Senoglu M. A case of pseudoaneurysm of the internal carotid artery following endoscopic endonasal pituitary surgery: Endovascular treatment with flow-diverting stent implantation. Acta Medica (Hradec Kralove). 2017. 60: 89-92
18. Kosty J, Pukenas B, Smith M, Storm PB, Zager E, Stiefel M. Iatrogenic vascular complications associated with external ventricular drain placement: A report of 8 cases and review of the literature. Neurosurgery. 2013. 72: ons208-213
19. Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000. 8: e4-
20. Le H, Munshi I, Macdonald RL, Wollmann R, Frank J. Traumatic aneurysm resulting from insertion of an intracranial pressure monitor. Case illustration. J Neurosurg. 2001. 95: 720-
21. Lee CH, Chen SM, Lui TN. Posterior cerebral artery pseudoaneurysm, a rare complication of pituitary tumor transsphenoidal surgery: Case report and literature review. World Neurosurg. 2015. 84: 1493.e1-3
22. Li LM, Timofeev I, Czosnyka M, Hutchinson PJ. Review article: The surgical approach to the management of increased intracranial pressure after traumatic brain injury. Anesth Analg. 2010. 111: 736-48
23. McLaughlin MR, Wahlig JB, Kaufmann AM, Albright AL. Traumatic basilar aneurysm after endoscopic third ventriculostomy: Case report. Neurosurgery. 1997. 41: 1400-3
24. Misaki K, Uchiyama N, Hayashi Y, Hamada J. Intracerebral hemorrhage secondary to ventriculoperitoneal shunt insertion--four case reports. Neurol Med Chir (Tokyo). 2010. 50: 76-9
25. Overton MC, Calvin TH. Iatrogenic cerebral cortical aneurysm. Case report. J Neurosurg. 1966. 24: 672-5
26. Pare L, Delfino R, Leblanc R. The relationship of ventricular drainage to aneurysmal rebleeding. J Neurosurg. 1992. 76: 422-7
27. Rayes M, Bahgat DA, Kupsky WJ, Mittal S. Middle cerebral artery pseudoaneurysm formation following stereotactic biopsy. Can J Neurol Sci. 2008. 35: 664-8
28. Raygor KP, Mooney MA, Snyder LA, Levitt MR, Albuquerque FC, Spetzler RF. Pseudoaneurysm of distal anterior cerebral artery branch following external ventricular drain placement. Oper Neurosurg (Hagerstown). 2016. 12: 77-82
29. Sahrakar K, Boggan JE, Salamat MS. Traumatic aneurysm: A complication of stereotactic brain biopsy: Case report. Neurosurgery. 1995. 36: 842-6
30. Shah KJ, Jones AM, Arnold PM, Ebersole K. Intracranial pseudoaneurysm after intracranial pressure monitor placement. J Neurointerv Surg. 2016. 8: e3-
31. Shirane R, Kondo T, Yoshida YK, Furuta S, Yoshimoto T. Ruptured cerebral pseudoaneurysm caused by the removal of a ventricular catheter. Case report. J Neurosurg. 1999. 91: 1031-3
32. Skandalakis GP, Korfias S, Kalyvas AV, Anagnostopoulos C, Sakas DE. A giant pseudoaneurysm of the occipital artery. Surg Neurol Int. 2017. 8: 281-
33. Srinivasan VM, Karas PJ, Sen AN, Fridley JS, Chen SR, Gopinath SP. Occipital artery pseudoaneurysm after posterior fossa craniotomy. World Neurosurg. 2017. 98: 868.e1-868.e4
34. Tubbs RS, Acakpo-Satchivi L, Blount JP, Oakes WJ, Wellons JC. Pericallosal artery pseudoaneurysm secondary to endoscopic-assisted ventriculoperitoneal shunt placement. Case report. J Neurosurg. 2006. 105: 140-2
35. White JC, Sayre GP, Whisnant JP. Experimental destruction of the media for the production of intracranial arterial aneurysms. J Neurosurg. 1961. 18: 741-5