- Department of Neurosurgery, Hiroshima University, Hiroshima, Japan,
- Department of Neurosurgery, University of Iowa Hospitals and Clinics, Iowa, United States.
Correspondence Address:
Kiyoharu Shimizu, Department of Neurosurgery, Hiroshima University, Hiroshima, Japan.
DOI:10.25259/SNI_1104_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kiyoharu Shimizu1, Takafumi Mitsuhara1, Masaaki Takeda1, Satoshi Yamaguchi2. Imaging evolution from “presyrinx” to syrinx in patient with spinal lipoma. 30-Dec-2021;12:633
How to cite this URL: Kiyoharu Shimizu1, Takafumi Mitsuhara1, Masaaki Takeda1, Satoshi Yamaguchi2. Imaging evolution from “presyrinx” to syrinx in patient with spinal lipoma. 30-Dec-2021;12:633. Available from: https://surgicalneurologyint.com/surgicalint-articles/11307/
Abstract
Background: The evolution of syrinx formation has rarely been documented. Here, we report a patient whose “presyrinx” evolved on successive magnetic resonance (MR) images to a mature syrinx.
Case Description: A patient had a lipoma and tethered cord at birth. At 3 weeks of age, he had undergone a partial removal of the lipoma and untethering of the spinal cord. At age 6, the thoracic MR images showed edema within the gray matter of the cord at the T7 level, consistent with a “presyrinx.” In addition, subsequent MR studies (i.e., at age 7) showed a small cavity in the right posterior horn of the cord accompanied by further expansion throughout the right-sided gray matter. Despite repeated cord untethering at age 7, the T7 parenchymal cord change evolved into a mature syrinx by age 10.
Conclusion: An infant with a lipoma/tethered cord, despite two instances of cord detethering (i.e., ages 3 weeks and 7 years), showed continued MR evolution of a “presyrinx” to a mature syrinx by age 10.
Keywords: Presyrinx, Spinal lipoma, Syrinx
INTRODUCTION
A syrinx is a fluid-filled cystic cavitation in the spinal cord parenchyma that develops due to the disturbed resorption of the extracellular fluid (ECF) from the spinal cord into the venous system.[
CASE REPORT
An infant presented with lipomyelomeningocele/tethered cord in the lumbosacral area at birth [
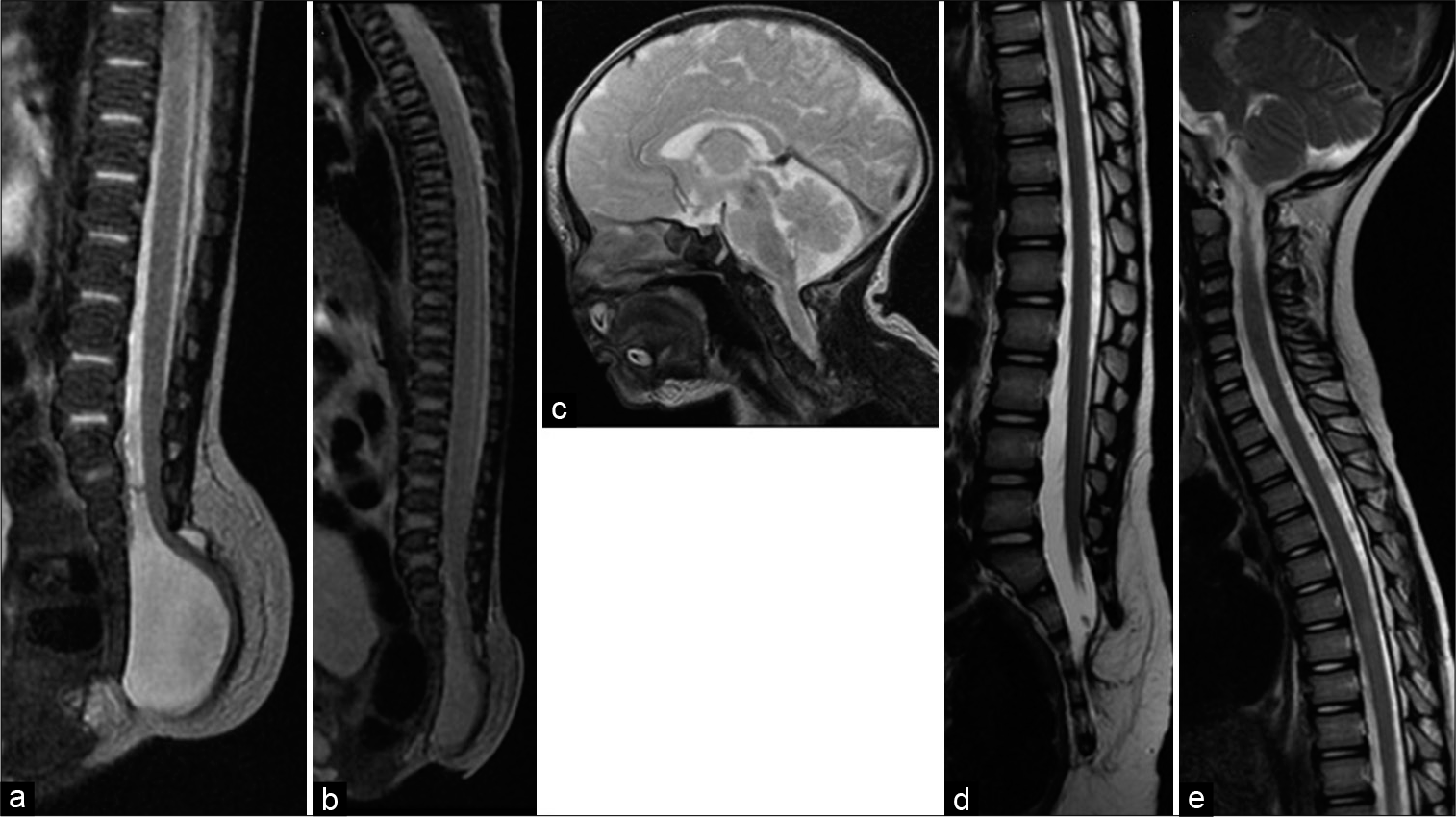
Figure 1:
(a) Sagittal T2-weighted magnetic resonance (MR) image at birth showed the sacral lipomyelomeningocele. The spinal cord prolapsed from the sacral epidural space and tethered at the subcutaneous fat. (b and c) No obvious syringomyelia, Chiari malformation, and hydrocephalus were observed at birth. (d and e) Sagittal T2-weighted MR image at age 5 years showed no syrinx formation. Although the low-lying conus could be observed, the patient was asymptomatic; therefore, close observation was continued.
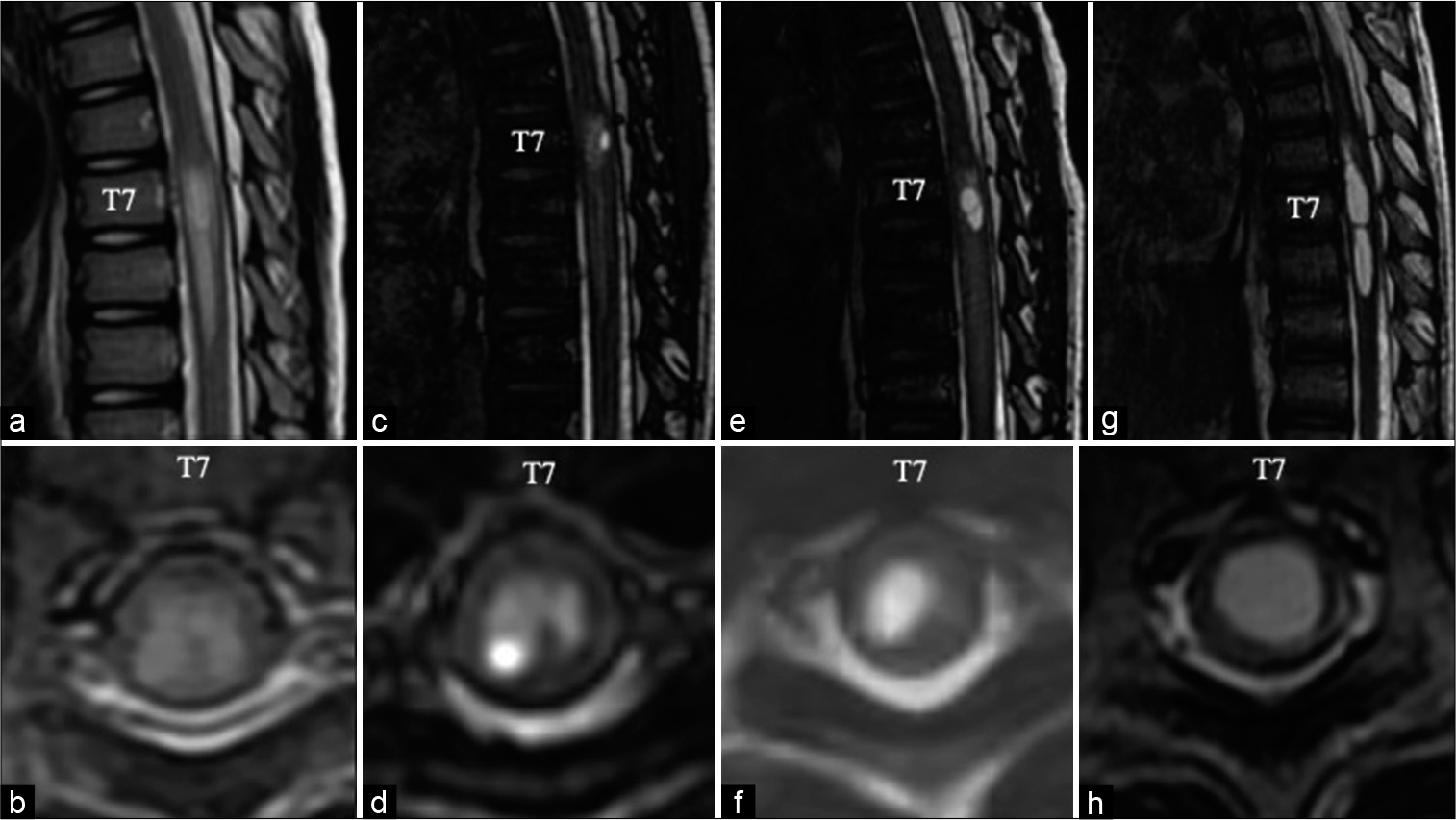
Figure 2:
(a) Sagittal T2-weighted magnetic resonance (MR) image at age 5 years showing the newly emerging T2 prolongation at T7-T9. (b) Axial T2-weighted MR image showing the abnormal T2 prolongation confined to the gray matter with no frank cavitation. At this time, the patient was still asymptomatic. (c and d) Six months later, the patient complained of intermittent pain and weakness in his lower limbs. MR image showing syringomyelia cavitation in the right posterior horn at T7. (e and f) Magnetic resonance (MR) image at age 6 years showing that the syrinx was enlarged and expanded throughout the right gray matter. Since retethering was considered the cause of the intermittent lower limb symptoms and the progression of syringomyelia, a revision untethering surgery was performed. After the operation, symptoms were resolved. (g and h) However, on MR imaging 6 months after surgery, the syrinx had enlarged into the entire spinal parenchyma.
DISCUSSION
Presyrinx state
“Presyrinxes” develop due to various disorders in the cerebrospinal fluid (CSF) circulation; Chiari Type I malformations, meningitis, basal arachnoiditis, cervical spondylosis, hydrocephalus, and posterior fossa arachnoid cysts.[
Syrinx formation attributed to ECF accumulation
Syrinxes occur due to disturbed ECF circulation/ accumulation in the spinal cord attributed to scarring of the arachnoid and traction of the spinal cord (i.e., the dorsal cord is most susceptible because of its anatomy).[
CONCLUSION
Chronological imaging from birth to 10 years of age showed progression of a “presyrinx” to a mature syrinx in a patient who as an infant presented with a lipomyelomeningocele and tethered cord.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwalla PK, Dunn IF, Scott RM, Smith ER. Tethered cord syndrome. Neurosurg Clin N Am. 2007. 18: 531-47
2. Akiyama Y, Koyanagi I, Yoshifuji K, Murakami T, Baba T, Minamida Y. Interstitial spinal-cord oedema in syringomyelia associated with Chiari Type 1 malformations. J Neurol Neurosurg Psychiatry. 2008. 79: 1153-8
3. Cheng S, Stoodley MA, Wong J, Hemley S, Fletcher DF, Bilston LE. The presence of arachnoiditis affects the characteristics of CSF flow in the spinal subarachnoid space: A modelling study. J Biomech. 2012. 45: 1186-91
4. Fischbein NJ, Dillon WP, Cobbs C, Weinstein PR The. “presyrinx” state: Is there a reversible myelopathic condition that may precede syringomyelia?. Neurosurg Focus. 2000. 8: E4
5. Greitz D. Unraveling the riddle of syringomyelia. Neurosurg Rev. 2006. 29: 251-63
6. Koyanagi I, Houkin K. Pathogenesis of syringomyelia associated with Chiari Type 1 malformation: review of evidences and proposal of a new hypothesis. Neurosurg Rev. 2010. 33: 271-84
7. Miyasaka K, Asano T, Ushikoshi S, Hida K, Koyanagi I. Vascular anatomy of the spinal cord and classification of spinal arteriovenous malformations. Interv Neuroradiol. 2000. 6: 195-8
8. Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997. 86: 483-92
9. Tsitouras V, Sgouros S. Syringomyelia and tethered cord in children. Childs Nerv Syst. 2013. 29: 1625-34