- Department of Neurosurgery, University of Occupational and Environmental Health, Kitakyusyu, Japan
Correspondence Address:
Shohei Nagasaka, Department of Neurosurgery, University of Occupational and Environmental Health, Kitakyusyu, Japan.
DOI:10.25259/SNI_759_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Noriaki Nomura, Shohei Nagasaka, Kohei Suzuki, Junkoh Yamamoto. Imaging-tracked progression of primary leptomeningeal gliomatosis: A case report. Surg Neurol Int 08-Nov-2024;15:411
How to cite this URL: Noriaki Nomura, Shohei Nagasaka, Kohei Suzuki, Junkoh Yamamoto. Imaging-tracked progression of primary leptomeningeal gliomatosis: A case report. Surg Neurol Int 08-Nov-2024;15:411. Available from: https://surgicalneurologyint.com/surgicalint-articles/13203/
Abstract
Background: Primary leptomeningeal gliomatosis (PLG) is a rare neoplasm characterized by the diffuse spread of glial tumor cells throughout the leptomeninges without any evidence of a primary tumor source in the brain or spinal cord parenchyma. Here, we present a case of PLG potentially linked to prior interventional radiotherapy.
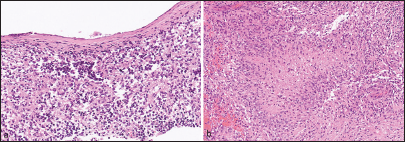
Case Description: The patient was a 75-year-old woman with a history of interventional radiology for a left internal carotid cavernous sinus fistula 13 years before presentation. Routine follow-up fluid-attenuated inversion recovery magnetic resonance imaging revealed a high intensity region spreading from the deep white matter of the subventricular zone (SVZ) to the insular cortex and medial temporal lobe. Subsequently, contrast-enhanced T1-weighted imaging revealed an enhanced effect consistent with extensive leptomeninges extending from the basilar cistern to the left Sylvian fissure. The patient underwent surgery, and subsequently histological examination of extracted tissue revealed a glioblastoma (GBM). Despite postoperative concurrent chemoradiotherapy and adjuvant temozolomide chemotherapy, the tumor increased in size, and the patient died 2 months postoperatively.
Conclusion: This case highlights the importance of careful follow-up and early therapeutic intervention in PLG, as it can be difficult to diagnose leptomeningeal lesions alone. This case also raises the possibility of radiation-induced GBM, and the criteria for diagnosis were fully met. The progression of PLG from the SVZ to the leptomeningeal site was tracked using imaging, providing valuable insights into the pattern of spread of this rare condition.
Keywords: Glioblastoma, Primary leptomeningeal gliomatosis, Radiation-induced glioblastoma, Subventricular zone
INTRODUCTION
Leptomeningeal diseases are known complications of metastatic cancers, including melanoma and breast, lung, and gastrointestinal cancer. Primary central nervous system (CNS) tumors, lymphoma, leukemia, and multiple myeloma are all common neoplastic tumors associated with leptomeningeal disease.[
CASE DESCRIPTION
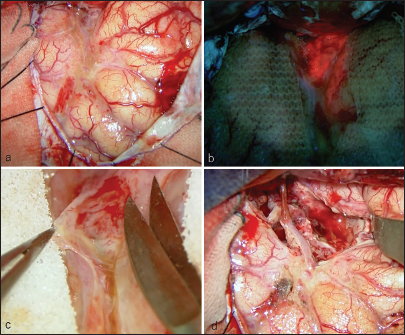
The patient was a 75-year-old woman with a history of interventional radiology (IVR) for a left internal carotid cavernous sinus fistula (CCF) 13 years before presentation [
Figure 1:
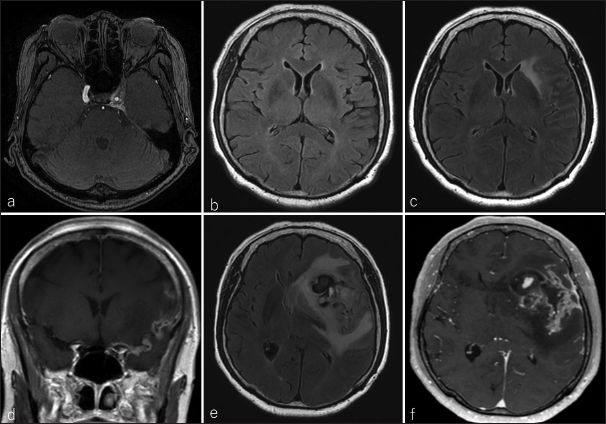
(a) Magnetic resonance angiography in the axial view revealed a left internal carotid cavernous sinus fistula (CCF) 13 years before the presentation; (b) imaging 1 year before presentation showed no occurrence of CCF or abnormal signal in the left subventricular zone (SVZ). (c) At presentation, the SVZ was highly intense on fluid-attenuated inversion recovery (FLAIR), and (d) extensive leptomeninges from the basiler cistern to the left Sylvian fissure could be observed on contrast-enhanced T1 weighted imaging (CE-T1WI). After 2 months, the tumor was enlarted and bled on (e) FLAIR and (f) CE-T1WI images.
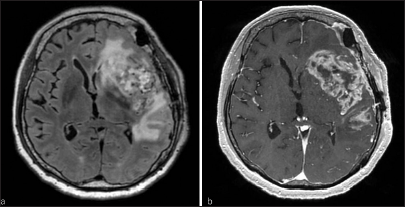
After 2 months, the patient was admitted due to motor aphasia and right hemiparesis, at which point an MRI revealed a contrast-enhanced, enlarged leptomeningeal lesion [
DISCUSSION
PLG is defined as a neoplasm confined to the meninges without any evidence of a primary tumor in the brain or spinal cord parenchyma, which develops in heterotopic glial cells within the meninges. Heterotopic glial cells can be observed in up to 1% of the normal population but have been noted in up to 25% of those with coexisting CNS malformations.[
In our case, the patient underwent MRI annually, and after 13 years of treatment, the CCF showed a high signal on FLAIR of the SVZ. CE-T1WI revealed no obvious intraparenchymal tumor but did indicate leptomeningeal enhancement. Vasculitis and metastatic lesions were considered differential diagnoses; however, blood tests and CT revealed no associated abnormalities. There are three diagnostic criteria for PLG: (1) no attachment of the tumor to the brain parenchyma, (2) no evidence of intra-axial lesions, and (3) leptomeningeal encapsulation of the tumor.[
Radiation-induced neoplasms of the CNS are rare and include meningiomas, sarcomas, gliomas, and gliosarcomas. Gliomas are the second most frequently identified radiation-induced CNS neoplasm.[
There have been no prior reports of radiation-induced GBM extending to the leptomeningeal dissemination. However, studies have indicated that glioma cells migrate from the initial site along the brain vessels to the subpial, subarachnoid, and subependymal spaces and tend to invade individuals or small groups in distant territories and abuse pre-existing supply lines.[
CONCLUSION
PLG is thought to be caused by ectopic glial cells. However, radiation-induced GBM can occur in the SVZ and spread to the leptomeningeal site, resulting in PLG, as in the present case. Because PLG can present during the developmental process, as in the present case, careful follow-up and early therapeutic intervention are important.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Autran D, Barrie M, Matta M, Monserrat C, Campello C, Petrirena G. Leptomeningeal gliomatosis: A single institution study of 31 patients. Anticancer Res. 2019. 39: 1035-41
2. Bathla G, Gupta S, Moritani T. Primary leptomeningeal glioblastoma with systemic metastases-case report and review of literature. Clin Imaging. 2015. 39: 672-6
3. Battista F, Muscas G, Dinoi F, Gadda D, Della Puppa A. Ventricular entry during surgical resection is associated with intracranial leptomeningeal dissemination in glioblastoma patients. J Neurooncol. 2022. 160: 473-80
4. Birzu C, Tran S, Bielle F, Touat M, Mokhtari K, Younan N. Leptomeningeal spread in glioblastoma: Diagnostic and therapeutic challenges. Oncologist. 2020. 25: e1763-76
5. Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone: Report of eleven cases 1948. Cancer. 1998. 82: 8-34
6. Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: A guerilla war. Acta Neuropathol. 2007. 114: 443-58
7. Cooper IS, Kernohan JW. Heterotopic glial nests in the subarachnoid space; histopathologic characteristics, mode of origin and relation to meningeal gliomas. J Neuropathol Exp Neurol. 1951. 10: 16-29
8. Hansen N, Wittig A, Hense J, Kastrup O, Gizewski ER, Van de Nes JA. Long survival of primary diffuse leptomeningeal gliomatosis following radiotherapy and temozolomide: Case report and literature review. Eu J Med Res. 2011. 16: 415-9
9. Hill MD, Mackenzie I, Mason WP. Radiation-induced glioma presenting as diffuse leptomeningeal gliomatosis: A case report. J Neuro Oncol. 2001. 55: 113-6
10. Ishige S, Iwadate Y, Ishikura H, Saeki N. Primary diffuse leptomeningeal gliomatosis followed with serial magnetic resonance images. Neuropathology. 2007. 27: 290-4
11. Lee JW, Wernicke AG. Risk and survival outcomes of radiation-induced CNS tumors. J Neurooncol. 2016. 129: 15-22
12. Lepreux S, Sagnier S, Perez JT, Léger F, Sibon I, Vital A. Primary diffuse leptomeningeal gliomatosis: Do we miss the diagnosis?. Clin Neuropathol. 2017. 36: 222-6
13. Mistry AM, Kelly PD, Gallant JN, Mummareddy N, Mobley BC, Thompson RC. Comparative analysis of subventricular zone glioblastoma contact and ventricular entry during resection in predicting dissemination, Hydrocephalus, and survival. Neurosurgery. 2019. 85: E924-32
14. Narayan V, Savardekar A, Mohammed N, Patra DP, Georgescu MM, Nanda A. Primary focal intracranial leptomeningeal glioma: Case report and review of the literature. World Neurosurg. 2018. 116: 163-8
15. Nayar G, Ejikeme T, Chongsathidkiet P, Elsamadicy AA, Blackwell KL, Clarke JM. Leptomeningeal disease: Current diagnostic and therapeutic strategies. Oncotarget. 2017. 8: 73312-28
16. Yamanaka R, Hayano A, Kanayama T. Radiation-induced gliomas: A comprehensive review and meta-analysis. Neurosurg Rev. 2018. 41: 719-31
17. Yamasaki K, Yokogami K, Ohta H, Yamashita S, Uehara H, Sato Y. Case of primary diffuse leptomeningeal gliomatosis. Brain Tumor Pathol. 2014. 31: 177-81
18. Zhu M, Zheng J, Zhu Y, Wan H, Wu Y, Hong D. Diffuse leptomeningeal gliomatosis initially presenting with intraventricular hemorrhage: A case report and literature review. BMC Neurol. 2015. 15: 77