- Department of Neurological Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA
- Cancer Biology and Therapeutics: High-Impact Cancer Research Program, Harvard Medical School, Boston, MA 02115, USA
- Faculty of Medicine, American University of Beirut, Beirut, Lebanon
- Department of Neurosurgery, Neuroscience Research Center, Faculty of Medical Science, Lebanese University, Beirut, Lebanon
Correspondence Address:
Jawad Fares, Youssef Fares
Department of Neurosurgery, Neuroscience Research Center, Faculty of Medical Science, Lebanese University, Beirut, Lebanon
DOI:10.4103/sni.sni_366_18
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Jawad Fares, Mohamad Y. Fares, Youssef Fares. Immune checkpoint inhibitors: Advances and impact in neuro-oncology. 25-Jan-2019;10:9
How to cite this URL: Jawad Fares, Mohamad Y. Fares, Youssef Fares. Immune checkpoint inhibitors: Advances and impact in neuro-oncology. 25-Jan-2019;10:9. Available from: http://surgicalneurologyint.com/surgicalint-articles/9169/
Keywords: Immune checkpoint inhibitors, immunotherapy, T-cells, Nobel prize, James P. Allison, Tasuko Honjo, neuro-oncology
INTRODUCTION
The Nobel Assembly, consisting of 50 professors at the Karolinska Institutet, in Sweden, awarded the 2018 Nobel Prize in Physiology or Medicine jointly to James P. Allison and Tasuku Honjo for their discovery of cancer therapy by inhibition of negative immune regulation.[
Dr. James Allison is an American immunologist who holds the position of professor and chair of immunology at the University of Texas M.D. Anderson Cancer Center. Dr. Tasuku Honjo is a Japanese immunologist who is a professor of immunology at Kyoto University.[
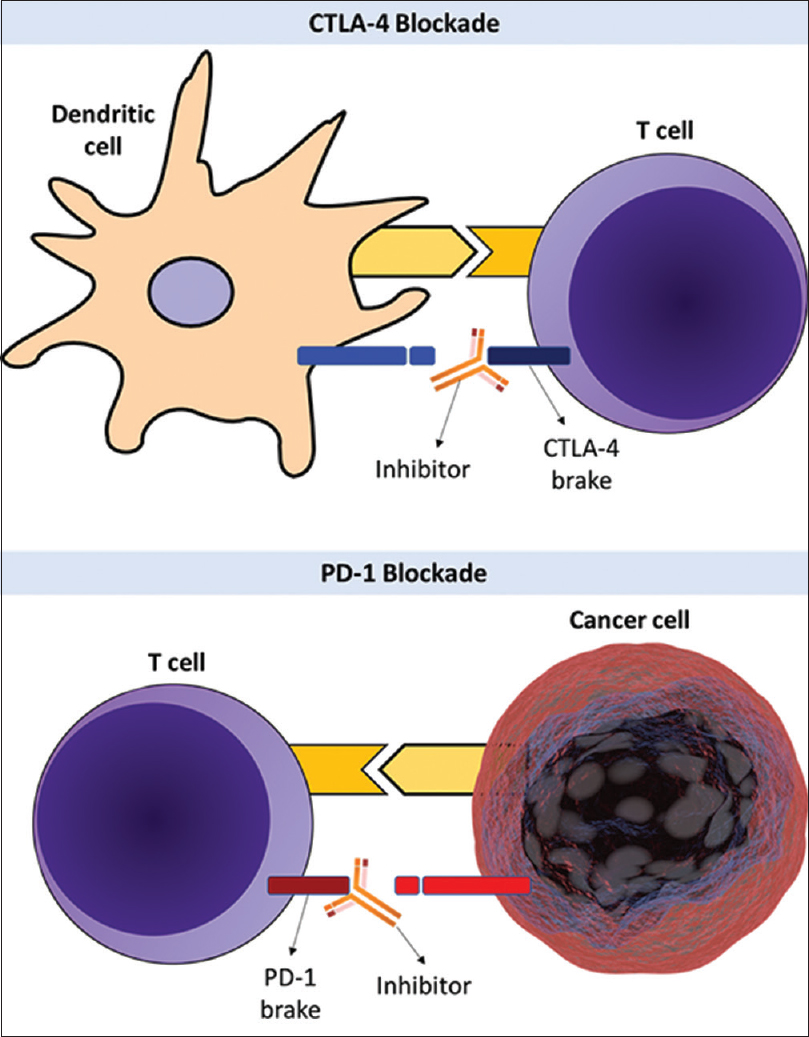
Figure 1
Upper half: cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) checkpoint protein functions as a brake on T-cells that inhibits T-cell activation. CTLA-4 inhibitors block the function of the brake leading to activation of T-cells and attack on cancer cells. Lower half: programmed cell death protein 1 (PD-1) is another checkpoint protein that functions as a brake that inhibits T-cell activation. PD-1 blockade inhibits the function of the brake leading to activation of T-cells and highly efficient attack on cancer cells
Clinical studies exploring the effects of CTLA-4 and PD-1 blockades have been dramatic. The treatment agents that are referred to as “immune checkpoint inhibitors,” have completely altered the outcome for certain groups of patients with advanced cancer. In tumors of the central nervous system (CNS) though, their effects remain to be seen. In this paper, we explore the impact of immune checkpoint inhibitors on CNS-related neoplasms and discuss the latest advances targeting CTLA-4 and PD-1 in neuro-oncology.
CTLA-4 TARGETTED IMMUNOTHERAPY
In 1996, James Allison, lead investigator in his laboratory at University of California, Berkeley, published in Science his observation that CTLA-4, a protein known as a target in the treatment of autoimmune diseases, is a negative regulator of T-cell activation.[
DISCOVERY OF PD-1
In 1992, 4 years before Allison's observations on CTLA-4 were published, Tasuko Honjo discovered PD-1 as a novel member of the immunoglobulin gene superfamily. His new observation published in The EMBO Journal suggested that the PD-1 protein may be involved in the classical type of programmed cell death.[
IMPACT IN NEURO-ONCOLOGY
The development of immune checkpoint inhibitors targeting CTLA-4 and PD-1 has significantly improved the treatment of a variety of cancers, such as metastatic melanoma, non-small cell lung cancer, and renal cell carcinoma. Nevertheless, little has been said about the effect of these inhibitors on CNS-related neoplasms.
Glioblastoma multiforme
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor (46%), as well as the deadliest.[
Preclinical studies corroborate that CTLA-4 blockade has shown positive results in animal models of GBM. After blockade of CTLA-4, there was an increase in number of CD4 T cells with improved function.[
PD-1 is highly expressed in GBM[
Metastatic brain tumors
Brain metastases outnumber primary malignant brain tumors with a ratio of 10 to 1.[
Studies have shown that immune checkpoint inhibitors are effective in the treatment of brain metastases from malignant melanoma and non-small cell lung cancer.[
Immune-related adverse events
Despite the effective antitumor immune response induced by these inhibitors, immune checkpoint blockade can result in inflammation of any organ. Inflammatory adverse effects that result from the treatment are known as immune-related adverse events. In general, PD-1 inhibitors have a lower incidence of immune-related adverse events compared with those that block CTLA-4. In addition, combination of nivolumab and ipilimumab has a higher rate of immune-related adverse events than either approach as monotherapy.[
Cost of therapy
Therapies with immune checkpoint inhibitors are quite expensive. The average annual cost of treatment with each drug can surpass $100,000. Managing the immune-related adverse events will also add to the tally. This makes it much harder to make decisions on the sequence of treatments and the dosing schedule. Policymakers must be informed about the value of these treatments to develop cost-effective strategies for therapy. For example, Kohn et al.[
CONCLUSION
The discovery and evolution of immune checkpoint inhibitors is one of the most exciting advances in cancer immunotherapy. Non-CNS tumors, specifically, have experienced impressive responses with long-lasting survival benefits. Early preclinical work has demonstrated that immunotherapy may potentially hold similar promise for GBM and metastatic brain cancers; however, more studies on the patient level are required to validate its true efficacy. As CNS tumors can develop multiple mechanisms for immune-resistance, combinations using multiple checkpoint inhibitors targeting both CTLA-4 and PD-1, with or without other immune-based strategies may be the most effective means in generating an antitumor immune response. In addition, discovering new checkpoint proteins and targeting the immune active microenvironment of CNS tumors can be vital to overcome potential resistance mechanisms. Awareness and multidisciplinary management of immune-related adverse events and developing cost-effective strategies for treatment are also necessary to ensure the optimal clinical benefit from these therapeutic agents.
References
1. Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012. 35: 385-9
2. Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014. 9: e101764-
3. Benson DM, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel, monoclonal anti-PD-1 antibody. Blood. 2010. 116: 2286-94
4. Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015. 17: 1064-75
5. Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013. 19: 3165-75
6. Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007. 13: 2158-67
7. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000. 192: 1027-34
8. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. 2016. 2: 1346-53
9. Hall W, Djalilian H, Nussbaum E, Cho K, Wa H. Long-term survival with metastatic cancer to the brain. Med Ontol. 2000. 17: 279-86
10. Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003. 100: 4712-7
11. Hodi FS, O’day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010. 363: 711-23
12. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992. 11: 3887-95
13. Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2004. 17: 133-44
14. Kohn CG, Zeichner SB, Chen Q, Montero AJ, Goldstein DA, Flowers CR. Cost-effectiveness of immune checkpoint inhibition in BRAF wild-type advanced melanoma. J Clin Oncol. 2017. 35: 1194-
15. Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997. 94: 8099-103
16. Lauko A, Thapa B, Jia X, Ahluwalia MS. Efficacy of immune checkpoint inhibitors in patients with brain metastasis from NSCLC, RCC, and melanoma. J Clin Oncol. 2018. 36: 214-214
17. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996. 271: 1734-6
18. Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015. 42: 587-600
19. Long GV, Atkinson V, Menzies AM, Lo S, Guminski AD, Brown MP. A randomized phase II study of nivolumab or nivolumab combined with ipilimumab in patients (pts) with melanoma brain metastases (mets): The Anti-PD1 Brain Collaboration (ABC). J Clin Oncol. 2017. 35: 9508-9508
20. Luksik AS, Maxwell R, Garzon-Muvdi T, Lim M. The role of immune checkpoint inhibition in the treatment of brain tumors. Neurotherapeutics. 2017. 14: 1049-65
21. Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: Distinct roles. J Immunol. 2007. 179: 7466-77
22. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012. 14: 48-54
23. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999. 11: 141-51
24. Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases: Histology, multiplicity, surgery, and survival. Cancer. 1996. 78: 1781-8
25. Reardon DA, Omuro A, Brandes AA, Rieger J, Wick A, Sepulveda J. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro Oncol. 2017. 19: iii21-
26. Solinas C, Porcu M, De Silva P, Musi M, Aspeslagh S, Scartozzi M. Cancer immunotherapy-associated hypophysitis. Semin Oncol. 2018. 45: 181-6
27. Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
28. Tang PrizeLast accessed on 2018 Oct 18. Available from: http://www.tang-prize.org/en/media_detail.php?cat=24&id=396.
29. Last accessed on 2018 Oct 18. Available from: https://www.nobelprize.org/prizes/ medicine/2018/summary/.
30. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012. 366: 2443-54
31. vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell–mediated glioma rejection. J Exp Med. 2013. 210: 2803-11
32. Wilmotte R, Burkhardt K, Kindler V, Belkouch MC, Dussex G, Tribolet Nd. B7-homolog 1 expression by human glioma: A new mechanism of immune evasion. Neuroreport. 2005. 16: 1081-5
33. Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R. Expression of the B7-related molecule B7-H1 by glioma cells: A potential mechanism of immune paralysis. Cancer Res. 2003. 63: 7462-7
34. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008. 8: 467-77