- Department of Neurology, San Juan Bautista School of Medicine, Caguas, Puerto Rico, USA.
- Caribbean Neurological Center, Guaynabo, USA.
- Department of Neurology, University of California, Los Angeles, California, USA.
Correspondence Address:
Sara Zarei
Caribbean Neurological Center, Guaynabo, USA.
DOI:10.25259/SNI_252_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sara Zarei, Irvin Maldonado, Laura Franqui-Dominguez, Cristina Rubi, Yanibel Tapia Rosa, Cristina Diaz-Marty, Guadalupe Coronado, Marimer C. Rivera Nieves, Golnoush Akhlaghipour, Angel Chinea. Impact of delayed treatment on exacerbations of multiple sclerosis among Puerto Rican patients. 11-Oct-2019;10:200
How to cite this URL: Sara Zarei, Irvin Maldonado, Laura Franqui-Dominguez, Cristina Rubi, Yanibel Tapia Rosa, Cristina Diaz-Marty, Guadalupe Coronado, Marimer C. Rivera Nieves, Golnoush Akhlaghipour, Angel Chinea. Impact of delayed treatment on exacerbations of multiple sclerosis among Puerto Rican patients. 11-Oct-2019;10:200. Available from: http://surgicalneurologyint.com/surgicalint-articles/9696/
Abstract
Background: There are limited data on multiple sclerosis (MS) patients in underserved groups, including Puerto Rico. In this study, we analyzed the characteristic of MS symptoms and number of relapses in Puerto Rican patients. We then compare these characteristics with MS patients from the US. The number of MS relapses is highly correlated with the treatment onset and adherence. Patients in Puerto Rico have been experiencing lengthy treatment delay. We will discuss the possible causes of such delay and its impact on MS prognosis.
Methods: This retrospective cohort study consisted of the evaluation of 325 medical records from MS patients attending the Caribbean Neurological Center from 2014 to 2019. We gathered symptoms and comorbidities data as binary objects. The treatment delay was calculated based on the mean value of days between diagnosis and treatment onset for these groups of patients.
Results: We found that on average, the treatment delay for MS patients in Puerto Rico (PR) to receive their medication was 120 days. The most common MS subtype was relapsing-remitting 72.8%, with a mean of 1.684 relapses per year. Initial symptoms were sensory 54%, visual 33.1%, motor 28.8%, coordination 23.2%, fatigue 9.7%, memory 7.3%, depression 6.5%, urinary 4.9%, gastrointestinal 2.4%, and sexual dysfunction 1.6%. The most common comorbidities were hypertension 18.4%, asthma 13.6%, and thyroid disease 12.8%. When we compared the comorbidities between the two populations, immune thrombocytopenia had the highest percent change with the value of almost 200% (0.001% of US patient vs. 0.8% of Puerto Rican MS patients).
Conclusion: Patients from Puerto Rico had a 33% higher relapse rate compared to the one reported for MS patients in the US. This higher rate may be related to the long delay in receiving their medications. They also had a higher rate of complex comorbidities such as immune thrombocytopenia or thyroid disease. Our findings provide a proof of concept that delay in receiving medications can increase the number of relapses and complex comorbidities among MS patients.
Keywords: Adverse impact of multiple sclerosis treatment delay, Multiple sclerosis comorbidities, Multiple sclerosis symptoms, Multiple sclerosis treatment, Puerto Rican multiple sclerosis patients, Puerto Rico health- care system
INTRODUCTION
Multiple sclerosis (MS) is a chronic, debilitating, and neurodegenerative autoimmune disease that attacks the central nervous system (CNS). The autoimmune reaction causes scarring (sclerosis) of the nerve and interruption of the conduction of nerve impulses from the brain along the spinal cord and to the rest of the body.
MS results in damaging the oligodendrocytes, which cover the axon of CNS neurons and therefore interferes with the neuronal transmission. The degree of demyelination, inflammation, and glial reaction that occurs in MS causes the variation in severity of the symptoms.[
The onset of symptoms occurs in individuals from 20 to 45 years of age and results in progressive neurological dysfunction. Almost two and a half million people worldwide and more than 700,000 individuals in the United States are living with MS with prevalence rates varying widely in different regions.[
The incidence and prevalence of MS are greater in higher latitudes farther from the equator and more frequent in females than in males with a ratio of 4:1.[
MS is incurable which makes the goal of the treatment to slow down the progression and alleviating the symptoms. Disease-modifying medications are used to reduce transient episodes of neurologic disability and limit the accumulation of focal white matter lesions on MRI.[
Corticosteroids have been also one of the most reliable first choices of treatment for several years, they have been useful in both accelerating recovery, and shortening the duration of relapse.[
Adherence is one of the most important factors to further increase the probability of treatment success in MS patients. A study from Al-Sabbagh et al. observed that patients with 90 or more days of gaps between therapies showed around two-fold greater risk of a severe relapse of MS.[
Some of the important factors that could cause the delay in the process of providing MS patients with appropriate therapy include insurance companies’ authorization process, availability of the drugs in local pharmacies, and lack of knowledge about MS disease among the health administration clerks. One of the most prevailing obstacles for both neurologists and MS patients in Puerto Rico is the long delay in receiving the medications. This could be also due to a lack of medical insurance coverage, making this one of the most important determinant factors that impact the overall health of patients with MS. This prolongation to start treatment leads to more exacerbations of their symptoms and an irreversible shift to a higher stage of the disease.[
Puerto Rico’s Government Health Care and Plans are constantly being reformed due to the economic situation in the Island, and this creates enormous issues when citizens seek medical assistance and treatment. In 2014, Puerto Rico had the highest health insurance coverage rate of about 94% while in the US mainland coverage at that time was about 88%. Even though a high percentage of Puerto Ricans had health insurance coverage, only 36% received insurance through private providers; the rest had publicly funded insurance.[
Once patients are diagnosed and approved to start the treatment, many other issues may arise. Many insurance companies will deny patients’ prescribed DMT due to insurance company preferring a more affordable drug than the one prescribed by the physician.[
In addition to the variation of quality of treatment, multiple studies show variations in prevalence, comorbidities, clinical, and demographic features of MS among different populations. Like many other autoimmune diseases, race and ethnicity are known as important factors on disease phenotype, clinical outcomes, and associated comorbidities.[
There is limited data about MS patients in underserved groups, including Puerto Rico. In this study, we analyzed Puerto Rican MS patient’s specific symptoms based on data provided by the Caribbean Neurological Center. Furthermore, we evaluated the exacerbation of their symptoms as the result of the delayed time in receiving their medications. Finally, we analyzed the wide variety of comorbidities associated with Puerto Rican MS patients. Our objective was to provide significant findings that will result in an earlier diagnosis as well as an immediate treatment option for MS patients. This study can serve as a stepping stone to adopt new methods of how to improve the quality of MS patients’ life by the early diagnosis as well as treatment.
METHODS
This retrospective cohort study focused on MS patients from Puerto Rico. The study sample consisted of 325 MS patients that visited the Caribbean Neurological Center when they were initially diagnosed with MS any time from the years 2014 to 2019. The electronic medical record contains demographic information, patient’s vital signs, medical history, family history, social history, recent and past prescribed medication, Expanded Disability Status Scale (EDSS), initial MS symptoms, number of relapses, Vitamin D3 status, MS subtypes, and diagnostic test results. Patient written consent was not required for this study since the datasets were without personal identifiers. The studied data did not include any identifiable information such as names, addresses, date of birth, social security numbers, driver’s license numbers, and telephone numbers. The study protocol was approved by the San Juan Bautista School of Medicine Institutional Review Board, Caguas, Puerto Rico.
The raw data were analyzed using R software. For all associated symptoms or comorbidities, data were collected as binary objects, “1” for the existence of a disease or symptom and “0” when it was not present in a patient. To obtain the amount of treatment delay, we calculated the average number of days between the time patients was diagnosed with MS and the time patient obtained the prescribed medications for all MS patients in our dataset. We also performed a comprehensive literature search on characteristics of MS patients from the US and created a table that compares their disease characteristics and comorbidities with MS patients in PR.
For MS patients from the US, we used multiple resources/ references in our tables to find the corresponding characteristics and comorbidities. However, for the graphs, we used the most recent study to compare the two populations.
RESULTS
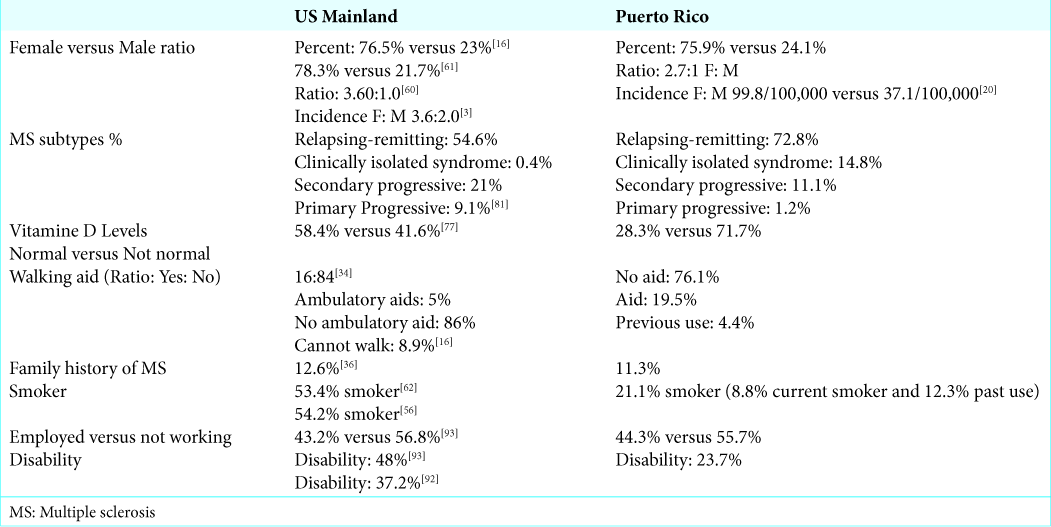
Our sample of 325 MS patients from the Caribbean Neurological Center database consisted of 75.9% females and 24.1% males. The average age at diagnosis among this population was 39 years ranging from a minimum of 15 to 64 years. We found that on average, it takes 120 days for MS patients in PR to receive their medication after they are diagnosed with MS.
We also evaluated common factors previously used for studying MS. These include MS subtypes, initial symptoms, family history of MS, Vitamin D deficiency; gait disturbance, smoking, working, and disability status of MS patients in PR.
MS subtypes and initial symptoms
The most common MS subtype among patients in PR is RR 72.8%, followed by CIS MS with 14.8%, secondary progressive with 11.1%, and primary progressive with 1.2%. The number of relapses among our MS patients ranged from 0 to 7 relapses per year; with a mean of 1.684/year and a median of 1 annually.
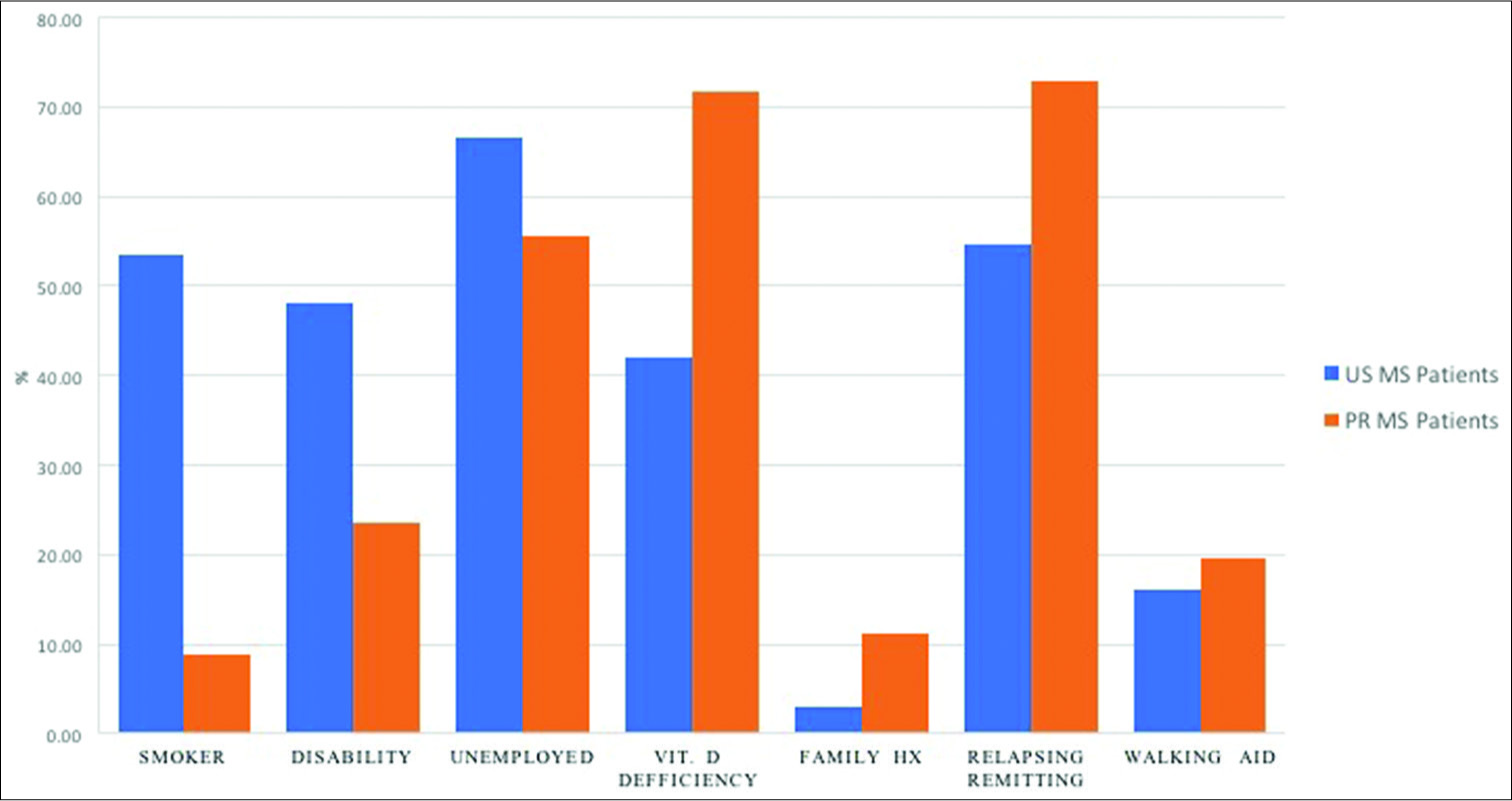
The first symptoms presented by our group of MS patients were predominantly sensory, visual and motor symptoms. The distribution of these initial symptoms was as follows: sensory 54%, visual 33.1%, motor 28.8%, coordination 23.2%, fatigue 9.7%, memory 7.3%, depression 6.5%, urinary 4.9%, gastrointestinal 2.4%, and sexual dysfunction 1.6%, as illustrated in
Family history of MS
One of the predisposing factors for MS is the existence of a history of MS among family members. Our study showed that 11.3% of our patients had a family history of MS. This includes either parents or siblings.
Vitamin D levels
Vitamin D deficiency (<30 ng/mL) was seen in 71.7% of PR MS patients. Previous studies also have demonstrated that Puerto Ricans have low Vitamin D levels, despite the high sun exposure.[
Gait, balance, and walking aid use
Use of a walking aid can be an indication of the amount of disability present in an MS patient. In fact, the use of walking assistance helps to determine the EDSS in these patients. In this group of MS patients, we found that 19.5% were using a walking aid, while 4.4% had previously used walker assistance. Therefore, 76.1% of our sample of PR MS patients can be considered fully ambulatory.
Smoking
Our study demonstrated that only 8.8% of patients are current smokers and 12.3% have a previous smoking history. Hence, a total of only 21.1% of PR patients were smokers sometime in their lifetime.
Working and disability status of patients
Our analysis demonstrates that 44.3% of Puerto Rican MS patients are working, 23.7% are disabled, and 4.4% are retired. This suggests that there is a large portion of the population that is not working (55.7%) and not receiving any benefits.
Comorbidities
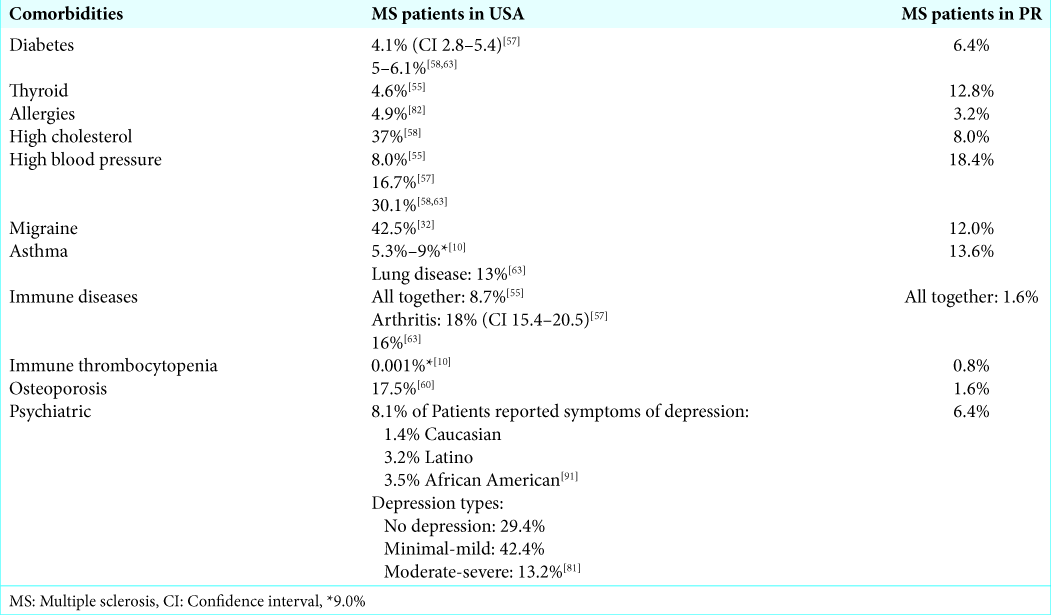
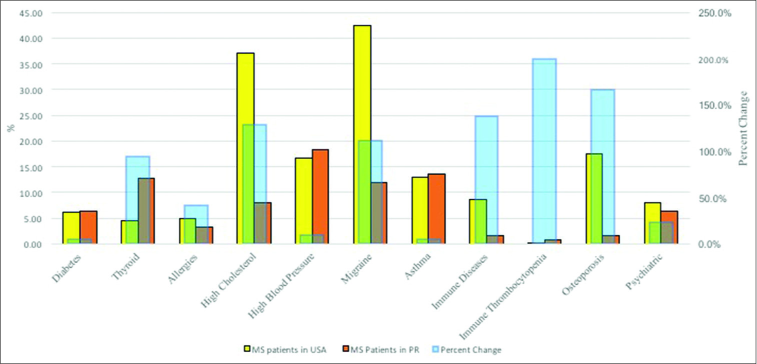
An analysis of comorbidities among our population of MS patients in PR showed that the most common condition is hypertension (18.4%). Other conditions observed that were above 10% were: asthma (13.6%), thyroid disease (12.8%), and migraine headaches (12%).
DISCUSSION
In this study, we found a distribution of 75.9% females and 24.1% males among the 325 MS patients evaluated from the Caribbean Neurological Center database. These results are consonant with the fact that MS is more prevalent in females than males. Our ratio, 3/1, differs slightly from the one reported in several studies, ranging approximately 4:1.[
When looking for MS subtypes percent in the United States population, RRMS had the highest percent, with 54.6% in a study that reported five different subtypes.[
The discrepancy might be attributed to the fact that the data were collected at the moment of diagnosis. RR is characterized by a surge of symptoms followed by recuperation and stability. In both the US and PR, it is shown that the more aggressive forms of MS are less common among these population.
In terms of the first symptoms of MS that our patients presented, sensory, visual, and motor symptoms were predominant. Stoppe et al. evaluated the symptoms their patients presented in relapses, and they are similar to the initial symptoms that our patients presented. They reported: sensory 42%, motor 29.4%, and visual 24.4% in all documented relapses.[
The initial symptoms of our MS patients were mostly a combination of the typical common symptoms such as optic, sensory, motor, brainstem, and cerebellar signs.[
MS has not been shown to be a genetic disease, though it is common to see multiple family members suffering from MS. In fact, it has been reported that there is a 15%– 20% risk of having a family member also affected with the disease.[
Analysis of Vitamin D levels is important in MS patients because it has been demonstrated to be a risk factor for developing MS.[
Moreover, in terms of walking aid use in our study, we found that 76.1% never used one while 19.5% were using assistance and 4.4% used in the past. Around 75% of MS patients will experience gait and balance problems throughout their lives.[
These numbers (in both PR and US) are very low in comparison with the percentage in Australia (48%) and United Kingdom (74%) reported in the same study.[
Lifestyle of the patients affects the course of the diseases, and smoking is an important variable to MS progression. Our study demonstrated that the smoking status of our subjects was 78.9% never smoke, 8.8% current smokers, and 12.3% smoked in the past. Hence, a total of 21.1% of our MS patients were cigarette smokers. This is much lower than reported in the US (53.4%).[
Marrie et al. reported smoking increases the risk of developing autoimmune comorbidities in MS patients (hazard ratio: 1.23; 95% CI: 1.08–1.41).[
MS can progress as a severely debilitating disease that affects the CNS causing symptoms such as fatigue, pain in multiple sites, and impairment of upper and lower limb function, among others.[
Studies have shown that many patients live constantly exiting and re-entering to the workforce depending on their disease progression and the overall impact on their body functionality.[
A study that grouped responses from Canadian and United States patients reported that employment was higher in RRMS patients (43.1%) in comparison with PPMS patients (18.1%).[
Based on our results, only 44.3% of PR MS patients were employed. Thus, we have a much lower percentage of employment among this population compared with MS patients from the US. The low employment status of the PR patients could be attributed to the Islands high unemployment rate since it is shown that only 23.7% of patients are disabled while in the US, the number is a much higher 48% disability rate.[
It is important to acknowledge that the patients were classified as disabled if before the interview they had been granted social security benefits. Approximately 70% of the Puerto Rican applicants will be denied each year, and the process may take from 4 months up to 2 years depending on the number of denials and appeals.[
The employment ratio by race in the US is as follows: C 47.5%, L 46.9%, and AA with 38.6%.[
Comorbidities play an important role in the progression of MS. As the patient grows older, the more comorbidity he/ she will have. Diseases such as hypertension, diabetes, and hyperlipidemia are among the most common.[
Comparing our results with the MS comorbidities of the US population were a bit challenging since the US data stems from different sources and include different races and ethnic groups. However, as shown in
Diabetes
Our result indicates that 6.4% of our MS patients had diabetes. This was slightly higher from what has been reported among MS patients in the US. In 2014 according to the Behavior and Risk Factor Surveillance System, 15.7% of Puerto Rican population were diabetic while the corresponding percentage in US was only 10.1% at that time. This shows in general, Puerto Rican has a higher risk of diabetes in comparison to US.[
In MS patients that suffer from diabetes, the treatment cost can be greater than MS patients with any other comorbidity. This is due to the substantially higher lifetime medical expenditures associated with diabetes and preventing its health complications.[
Hou et al. published a study in 2017, demonstrating a significant association between diabetes mellitus type 2 and MS incidence.[
Thyroid disorders
Our result shows that 12.8% of the Puerto Rican MS patients studied had thyroid disease. On the other hand, only 4.6% of MS patients in the US were found with thyroid disorders.[
Thyroiditis among other autoimmune diseases has been found to influence changes on the brain in MS patients.[
Hyperlipidemia
High cholesterol was found in 37% of the US patients that reported comorbidities to the NARCOMS.[
Hypertension
Multiple studies have reported that 8%–30.1% of MS patients in the US are suffering from hypertension.[
Migraines/headaches
In a meta-analysis performed by Foley et al., the prevalence of pain due to headache was found in 42.5% (95% CI 33.2%– 52.1%) of the patients suffering from all MS subtypes.[
Among our patients, 12% presented with migraine headache. This is lower compared to MS patient from the US. This discrepancy could be explained by the fact that the prevalence of migraine headache is lower among PR general population compared to the US. According to a study by Miranda et al., prevalence of migraine headache among Puerto Rican was 13%. Hence, MS patients carry similar prevalence rate of migraine headache as the general population in PR.
Asthma/lung disease
Pulmonary diseases are common in MS patients. A meta- analysis from Europe and North America found a range of 0.5%–25.0% for asthma and a 0.62%–2.9% range for COPD in MS patients.[
Immune diseases
Autoimmune diseases were reported in 8.7% of the patients who responded to the 2006 questionnaire by NARCOMS in the US.[
Immune thrombocytopenia
Almost 1% of our patients presented with immune thrombocytopenia. This was higher than US patients, with only 0.001%. Marrie et al. reported that Alemtuzumab, one of the medications that patients are using to treat MS, can cause immune thrombocytopenia.[
Osteoporosis
In 2007, 27.2% of the MS population in North America reported low bone mass or osteoporosis.[
Psychiatric
Psychiatric disorders are common in patients suffering from MS around the world.[
As we mentioned earlier, on average, it takes 120 days for MS patients in PR to receive their medication after they are diagnosed with MS. Such delay in receiving medications can cause more relapses and complex comorbidities among Puerto Rican MS patients.
Consequences of delay in treatment
Our findings showed that the number of RRMS is higher among PR MS patients in comparison with patients from the US. In terms of relapses, the values from our study ranged from 0 to 7 relapses per year; with a mean of 1.684 per year and a median of 1 annually. On the other hand, in a study conducted by Olek, the frequency of relapses among MS patients in the US was 1.27/year.[
The number of relapses and neuronal disability progression in patients that are more adherent to their treatment has been reported by previous studies to be relatively lower in comparison with the patient that is not receiving treatment for any reason.[
The benefits of early treatment of MS have been proven in multiple studies and have shown to decrease the frequency of relapses and slowing progression of the disease-related disability.[
In a study by Adams et al., they interviewed elderly Puerto Ricans that are living in the United States mainland. According to their study, the main reason Puerto Rican decided to stay at mainland is due to having easier access to medical services and excess reliance on medications.[
Health care contributes to 20% of the Puerto Rican economy, but with the health care crisis it leads to less access to medical care and decreases health-care providers in the Island. Puerto Ricans have to pay Medicare taxes and Social Security; however, in comparison with other 50 states, they receive the least federal funding for health care.[
Puerto Rico’s medical services were even more disrupted considerably after natural disaster of Hurricane Maria in 2017. The inability to access medications has been the most frequently reported issue. In addition to treatment delays, lack of medical facilities and physicians are among other major issues that have been arising in Puerto Rico.[
Limitations
The date of treatment onset was not included in our patients’ electronic medical records. Hence, to obtain such information, patients’ files had to be accessed manually. Looking through paper medical files takes time specifically when there is large number of patients. We had to go through all the doctors’ handwritten notes carefully to find the actual start date of initial treatment. Looking over 325 files became at times dreary which caused this part to take more time than expected. Furthermore, to ensure data were gathered correctly we had more than one person to perform the manual data collection to ensure we have obtained the correct information. If the date of onset of treatment had been in an electronic format, the gathering of such information for this research could have been achieved more efficiently.
CONCLUSION
As previously mentioned, there are limited studies about MS in PR in this critical and growing patient population. However, with this study, we shed light onto the characteristics of the Puerto Rican MS patients and discussed the possible consequences of delay in receiving their medications. Patients from the Caribbean Neurological Center database that participated in this study were demographically similar to those reported in MS-related literature in terms of gender ratio, age of diagnosis, and initial symptoms. The comorbidities that were more predominant in our patients were hypertension, asthma, thyroid disease, and migraines. Immune thrombocytopenia was shown in higher rates among our patients compared to MS patients from the US. Only a few had a family history of MS and most of them reported low Vitamin D3 levels. In terms of MS subtypes, RRMS was first, followed by PRMS, SPMS, and finally by PPMS.
One of the significant issues that Puerto Rican MS patients are currently facing is the delay in receiving their medications. On average, it takes 120 days for MS patients in PR to receive their medication. Consonant with this finding, their average rate of relapse was 33% higher compared to the US. Puerto Rican patients also had a higher rate of complex comorbidities such as immune thrombocytopenia or thyroid disease which could be related to delayed treatment onset.
Insurance companies’ authorization process, local pharmacy availability of the drugs and lack of knowledge about MS disease among the administrative clerks all play important factors in delaying the process of providing the MS patients with their medication. This leads to more exacerbations of their symptoms and an irreversible shift to a higher stage of the disease. Given the fact that the majority of MS medications are costly, insurance companies require specific authorizations. Many insurance companies in PR have a lengthy process in issuing authorization for MS medications.
A comprehensive medical and cost analysis need to be performed to provide the health insurance companies and corresponding government agencies with sufficient evidence that delaying these authorization processes will have negative economic consequences.
Hence, our findings serve as a stepping stone for such analysis. To conduct a comprehensive analysis, we would need to collect data from all those patients that had experienced the delay in receiving their medications. Next, we will need to obtain the total aggregated cost as the result of the exacerbations of patients’ symptoms. This includes any hospital fees, doctor visit fees, and any additional medication that the patient had to purchase while waiting for the primary treatment to arrive.
Therefore, we could achieve a substantial positive impact on the economy and health-care system by utilizing proper management strategies that lead to less reliance on inpatient care while increasing access to medications at home. Such strategies will reduce the frequency and severity of MS relapses.
References
1. Adams WE, Todorova IL, Guzzardo MT, Falcón LM. The problem here is that they want to solve everything with pills: Medication use and identity among mainland puerto ricans. Sociol Health Illn. 2015. 37: 904-19
2. Al-Sabbagh A, Bennett R, Kozma C, Dickson M, Meletiche D. Medication gaps in disease-modifying drug therapy for multiple sclerosis are associated with an increased risk of relapse: Findings from a national managed care database. J Neurol. 2008. 255: 79-
3. Alschuler KN, Jensen MP, Ehde DM. Defining mild, moderate, and severe pain in persons with multiple sclerosis. Pain Med. 2012. 13: 1358-65
4. Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: The impact of depression, fatigue and disability. Mult Scler. 2001. 7: 340-4
5. Annunziata P, De Santi L, Di Rezze S, Millefiorini E, Capello E, Mancardi G. Clinical features of sjogren’s syndrome in patients with multiple sclerosis. Acta Neurol Scand. 2011. 124: 109-14
6. Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014. 71: 306-14
7. Ascherio A, Munger KL. Epidemiology of multiple sclerosis: From risk factors to prevention-an update. Semin Neurol. 2016. 36: 103-14
8. Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation United States, 2013-2015. MMWR Morb Mortal Wkly Rep. 2017. 66: 246-53
9. Barcellos LF, Kamdar BB, Ramsay PP, DeLoa C, Lincoln RR, Caillier S. Clustering of autoimmune diseases in families with a high-risk for multiple sclerosis: A descriptive study. Lancet Neurol. 2006. 5: 924-31
10. Bhargava P, Cassard S, Steele SU, Azevedo C, Pelletier D, Sugar EA. The Vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: Study design for a multicenter, randomized, double-blind controlled trial of Vitamin D in multiple sclerosis. Contemp Clin Trials. 2014. 39: 288-93
11. Bourdette DN, Hartung DM, Whitham RH. Practices of US health insurance companies concerning MS therapies interfere with shared decision-making and harm patients. Neurol Clin Pract. 2016. 6: 177-82
12. Buchanan RJ, Minden SL, Chakravorty BJ, Hatcher W, Tyry T, Vollmer T. A pilot study of young adults with multiple sclerosis: Demographic, disease, treatment, and psychosocial characteristics. Disabil Health J. 2010. 3: 262-70
13. Buchanan RJ, Zuniga MA, Carrillo-Zuniga G, Chakravorty BJ, Tyry T, Moreau RL. Comparisons of latinos, African Americans, and caucasians with multiple sclerosis. Ethn Dis. 2010. 20: 451-7
14. Campbell JD, Ghushchyan V, Brett McQueen R, Cahoon-Metzger S, Livingston T, Vollmer T. Burden of multiple sclerosis on direct, indirect costs and quality of life: National US estimates. Mult Scler Relat Disord. 2014. 3: 227-36
15. Capra R, Cordioli C, Rasia S, Gallo F, Signori A, Sormani MP. Assessing long-term prognosis improvement as a consequence of treatment pattern changes in MS. Mult Scler. 2017. 23: 1757-61
16. Cerqueira JJ, Compston DAS, Geraldes R, Rosa MM, Schmierer K, Thompson A. Time matters in multiple sclerosis: Can early treatment and long-term follow-up ensure everyone benefits from the latest advances in multiple sclerosis?. J Neurol Neurosurg Psychiatry. 2018. 89: 844-50
17. Chinea A, Pérez N, Perez-Canabal A, Rojas F, Torres J, Poser C. The Puerto Rico study for the prevalence of multiple sclerosis. Bol Asoc Med P R. 2012. 104: 4-9
18. Chinea A, Ríos-Bedoya CF, Rubi C, Vicente I, Estades ER, Hernandez-Silvestrini YG. Incidence of multiple sclerosis in Puerto Rico, 2014: A population-based study. Neuroepidemiology. 2017. 48: 55-60
19. Chinea A, Ríos-Bedoya CF, Vicente I, Rubí C, García G, Rivera A. Increasing incidence and prevalence of multiple sclerosis in Puerto Rico (2013-2016). Neuroepidemiology. 2017. 49: 106-12
20. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise Vitamin D3. Lancet. 1982. 1: 74-6
21. Conway DS, Thompson NR, Cohen JA. Influence of hypertension, diabetes, hyperlipidemia, and obstructive lung disease on multiple sclerosis disease course. Mult Scler. 2017. 23: 277-85
22. Corona T, Román GC. Multiple sclerosis in Latin America. Neuroepidemiology. 2006. 26: 1-3
23. Di Pauli F, Reindl M, Ehling R, Schautzer F, Gneiss C, Lutterotti A. Smoking is a risk factor for early conversion to clinically definite multiple sclerosis. Mult Scler. 2008. 14: 1026-30
24. Durand M. Available from: http://www.momentummagazineonline.com/ms-classifications-revised [Last accessed on 2019 Jan 29].
25. Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004. 3: 104-10
26. Eitzen A, Finlayson M, Carolan-Laing L, Nacionales AJ, Walker C, O’Connor J. The development of an observational screening tool to assess safe, effective and appropriate walking aid use in people with multiple sclerosis. Disabil Rehabil Assist Technol. 2017. 12: 641-6
27. . Available from: https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS [Last accessed on 2019 Mar 05].
28. Ernstsson O, Gyllensten H, Alexanderson K, Tinghög P, Friberg E, Norlund A. Cost of illness of multiple sclerosis a systematic review. PLoS One. 2016. 11: e159129-
29. Filippi M, Bozzali M, Rovaris M, Gonen O, Kesavadas C, Ghezzi A. Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain. 2003. 126: 433-7
30. Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil. 2006. 87: 1274-9
31. Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA, Chandran S. Prevalence and natural history of pain in adults with multiple sclerosis: Systematic review and meta-analysis. Pain. 2013. 154: 632-42
32. Gunn H, Cameron M, Hoang P, Lord S, Shaw S, Freeman J. Relationship between physiological and perceived fall risk in people with multiple sclerosis: Implications for assessment and management. Arch Phys Med Rehabil. 2018. 99: 2022-9
33. Hadjigeorgiou G, Dardiotis E, Tsivgoulis G, Doskas T, Petrou D, Makris N. Observational study assessing demographic, economic and clinical factors associated with access and utilization of health care services of patients with multiple sclerosis under treatment with interferon beta-1b (EXTAVIA). PLoS One. 2014. 9: e113933-
34. Harirchian MH, Fatehi F, Sarraf P, Honarvar NM, Bitarafan S. Worldwide prevalence of familial multiple sclerosis: A systematic review and meta-analysis. Mult Scler Relat Disord. 2018. 20: 43-7
35. Hedström AK, Katsoulis M, Hössjer O, Bomfim IL, Oturai A, Sondergaard HB. The interaction between smoking and HLA genes in multiple sclerosis: Replication and refinement. Eur J Epidemiol. 2017. 32: 909-19
36. Hou WH, Li CY, Chang HH, Sun Y, Tsai CC. A population-based cohort study suggests an increased risk of multiple sclerosis incidence in patients with Type 2 diabetes mellitus. J Epidemiol. 2017. 27: 235-41
37. .editors. How to Apply for Social Security Disability in Puerto Rico. p.
38. . International Multiple Sclerosis Genetics Consortium (IMSGC). The expanding genetic overlap between multiple sclerosis and Type I diabetes. Genes Immun. 2009. 10: 11-4
39. Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS study group. N Engl J Med. 2000. 343: 898-904
40. Joy JE, Johnston RB.editors. Institute of Medicine (US) Committee on Multiple Sclerosis: Current Status and Strategies for the Future. Characteristics and Management of Major Symptoms. Washington, DC: National Academies Press; 2001. p.
41. Julian LJ, Vella L, Vollmer T, Hadjimichael O, Mohr DC. Employment in multiple sclerosis. Exiting and re-entering the work force. J Neurol. 2008. 255: 1354-60
42. Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH. Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: A 3-year follow-up analysis of the BENEFIT study. Lancet. 2007. 370: 389-97
43. Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH. Treatment with interferon beta-1b delays conversion to clinically definite and mcDonald MS in patients with clinically isolated syndromes. Neurology. 2006. 67: 1242-9
44. Kishore N, Marqués D, Mahmud A, Kiang MV, Rodriguez I, Fuller A. Mortality in Puerto Rico after hurricane maria. N Engl J Med. 2018. 379: e30-
45. Kister I, Chamot E, Salter AR, Cutter GR, Bacon TE, Herbert J. Disability in multiple sclerosis: A reference for patients and clinicians. Neurology. 2013. 80: 1018-24
46. Ko J, Katic B, Simacek K, Moreton D, Buechler N, Varga S.editorsDisease-modifying Therapy Access Issues and their Impact on Multiple Sclerosis Patients: An Online Mixed Methods Study. p.
47. Kurtzke JF. Epidemiology and etiology of multiple sclerosis. Phys Med Rehabil Clin N Am. 2005. 16: 327-49
48. Libon F, Cavalier E, Nikkels AF. Skin color is relevant to Vitamin D synthesis. Dermatology. 2013. 227: 250-4
49. Lizak N, Lugaresi A, Alroughani R, Lechner-Scott J, Slee M, Havrdova E. Highly active immunomodulatory therapy ameliorates accumulation of disability in moderately advanced and advanced multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017. 88: 196-203
50. Lizán L, Comellas M, Paz S, Poveda JL, Meletiche DM, Polanco C. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: A review of the literature. Patient Prefer Adherence. 2014. 8: 1653-64
51. Lorefice L, Fenu G, Pitzalis R, Scalas G, Frau J, Coghe G. Autoimmune comorbidities in multiple sclerosis: What is the influence on brain volumes? A case-control MRI study. J Neurol. 2018. 265: 1096-101
52. Marck CH, Neate SL, Taylor KL, Weiland TJ, Jelinek GA. Prevalence of comorbidities, overweight and obesity in an international sample of people with multiple sclerosis and associations with modifiable lifestyle factors. PLoS One. 2016. 11: e148573-
53. Markowitz CE. Multiple sclerosis update. Am J Manag Care. 2013. 19: s294-300
54. Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler. 2008. 14: 1091-8
55. Marrie RA, Cohen J, Stuve O, Trojano M, Sørensen PS, Reingold S. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Mult Scler. 2015. 21: 263-81
56. Marrie RA, Cutter G, Tyry T, Campagnolo D, Vollmer T. Smoking status over two years in patients with multiple sclerosis. Neuroepidemiology. 2009. 32: 72-9
57. Marrie RA, Cutter G, Tyry T, Vollmer T. A cross-sectional study of bone health in multiple sclerosis. Neurology. 2009. 73: 1394-8
58. Marrie RA, Cutter GR, Tyry T, Cofield SS, Fox R, Salter A. Upper limb impairment is associated with use of assistive devices and unemployment in multiple sclerosis. Mult Scler Relat Disord. 2017. 13: 87-92
59. Marrie RA, Horwitz R, Cutter G, Tyry T. Cumulative impact of comorbidity on quality of life in MS. Acta Neurol Scand. 2012. 125: 180-6
60. Marrie RA, Horwitz RI, Cutter G, Tyry T, Vollmer T. Association between comorbidity and clinical characteristics of MS. Acta Neurol Scand. 2011. 124: 135-41
61. Marrie RA, Horwitz RI, Cutter G, Tyry T, Vollmer T. Smokers with multiple sclerosis are more likely to report comorbid autoimmune diseases. Neuroepidemiology. 2011. 36: 85-90
62. Marrie RA, Miller A, Sormani MP, Thompson A, Waubant E, Trojano M. Recommendations for observational studies of comorbidity in multiple sclerosis. Neurology. 2016. 86: 1446-53
63. Marrie RA, Reider N, Stuve O, Trojano M, Sorensen PS, Cutter GR. The incidence and prevalence of comorbid gastrointestinal, musculoskeletal, ocular, pulmonary, and renal disorders in multiple sclerosis: A systematic review. Mult Scler. 2015. 21: 332-41
64. Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010. 74: 1041-7
65. Marrie RA, Yu BN, Leung S, Elliott L, Caetano P, Warren S. Rising prevalence of vascular comorbidities in multiple sclerosis: Validation of administrative definitions for diabetes, hypertension, and hyperlipidemia. Mult Scler. 2012. 18: 1310-9
66. Mattei J, Tamez M, Ríos-Bedoya CF, Xiao RS, Tucker KL, Rodríguez-Orengo JF. Health conditions and lifestyle risk factors of adults living in Puerto Rico: A cross-sectional study. BMC Public Health. 2018. 18: 491-
67. Miranda MT, Suárez E, Abbas M, Chinea A, Tosado R, Mejías IA. HLA class I; II alleles in multiple sclerosis patients from Puerto Rico. Bol Asoc Med P R. 2013. 105: 18-23
68. Morran MP, Vonberg A, Khadra A, Pietropaolo M. Immunogenetics of Type 1 diabetes mellitus. Mol Aspects Med. 2015. 42: 42-60
69. Morrow SA, Drake A, Zivadinov R, Munschauer F, Weinstock-Guttman B, Benedict RH. Predicting loss of employment over three years in multiple sclerosis: Clinically meaningful cognitive decline. Clin Neuropsychol. 2010. 24: 1131-45
70. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006. 296: 2832-8
71. Newland P, Wagner JM, Salter A, Thomas FP, Skubic M, Rantz M. Exploring the feasibility and acceptability of sensor monitoring of gait and falls in the homes of persons with multiple sclerosis. Gait Posture. 2016. 49: 277-82
72. O’Connor RJ, Cano SJ, Ramió i Torrentà L, Thompson AJ, Playford ED. Factors influencing work retention for people with multiple sclerosis: Cross-sectional studies using qualitative and quantitative methods. J Neurol. 2005. 252: 892-6
73. Olek MJ.editorsDisease-modifying Treatment of Relapsing-remitting Multiple Sclerosis in Adults. p.
74. Olivera EJ, Palacios C. Use of supplements in puerto rican older adults residing in an elderly project. P R Health Sci J. 2012. 31: 213-9
75. Paparrigopoulos T, Ferentinos P, Kouzoupis A, Koutsis G, Papadimitriou GN. The neuropsychiatry of multiple sclerosis: Focus on disorders of mood, affect and behaviour. Int Rev Psychiatry. 2010. 22: 14-21
76. Patti F. Optimizing the benefit of multiple sclerosis therapy: The importance of treatment adherence. Patient Prefer Adherence. 2010. 4: 1-9
77. Perreira K, Peters R, Cafarella Lallemand N, Stephen Z.editors. Puerto Rico Health Care Infrastructure Assessment: Site Visit Report. Puerto Rico. 2017. p.
78. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European study group on interferon beta-1b in secondary progressive MS. Lancet. 1998. 352: 1491-7
79. Polman CH. Medicine Cabinet. US Patent, No. 4,663,621. 2000. 173: 398-402
80. Ramírez-Vick M, Hernández-Dávila L, Rodríguez-Rivera N, López-Valentín M, Haddock L, Rodríguez-Martínez R. Prevalence of Vitamin D insufficiency and deficiency among young physicians at university district hospital in san juan, Puerto Rico. P R Health Sci J. 2015. 34: 83-8
81. Rao SM, Leo GL, Ellington L, Nauertz T, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. Neurology. 1991. 41: 692-6
82. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018. 378: 169-80
83. Reider N, Salter AR, Cutter GR, Tyry T, Marrie RA. Potentially modifiable factors associated with physical activity in individuals with multiple sclerosis. Res Nurs Health. 2017. 40: 143-52
84. Rico P, Care H, Perreira K, Peters R, Lallemand N, Zuckerman S.editors. Rese Arch Report Site Visit Report. 2017. p.
85. Roessler RT, Rumrill PD, Li J, Daly K, Anhalt K. High-priority employment concerns of hispanics/latinos with multiple sclerosis in the United States. J Vocat Rehabil. 2016. 45: 121-31
86. Roman J. The Puerto Rico healthcare crisis. Ann Am Thorac Soc. 2015. 12: 1760-3
87. Salter A, Thomas N, Tyry T, Cutter G, Marrie RA. Employment and absenteeism in working-age persons with multiple sclerosis. J Med Econ. 2017. 20: 493-502
88. Santa ML.editors. Diabetes Mellitus in Puerto Rico: Opportunities to be Discovered Ciencia Puerto Rico. Cienc. Puerto Rico. 2016. p.
89. Sayre RM, Dowdy JC. Darkness at noon: Sunscreens and Vitamin D3. Photochem Photobiol. 2007. 83: 459-63
90. Smith MM, Arnett PA. Factors related to employment status changes in individuals with multiple sclerosis. Mult Scler. 2005. 11: 602-9
91. Stoppe M, Busch M, Krizek L, Then Bergh F. Outcome of MS relapses in the era of disease-modifying therapy. BMC Neurol. 2017. 17: 151-
92. Tanasescu R, Constantinescu CS, Tench CR, Manouchehrinia A. Smoking cessation and the reduction of disability progression in multiple sclerosis: A cohort study. Nicotine Tob Res. 2018. 20: 589-95
93. Tettey P, Simpson S, Taylor BV, van der Mei IA. The co-occurrence of multiple sclerosis and Type 1 diabetes: Shared aetiologic features and clinical implication for MS aetiology. J Neurol Sci. 2015. 348: 126-31
94. Van Dyne EA, Neaterour P, Rivera A, Bello-Pagan M, Adams L, Munoz-Jordan J. Incidence and outcome of severe and nonsevere thrombocytopenia associated with zika virus infection-Puerto Rico, 2016. Open Forum Infect Dis. 2019. 6: ofy325-
95. Vijayasingham L, Jogulu U, Allotey P. Work change in multiple sclerosis as motivated by the pursuit of illness-work-life balance: A qualitative study. Mult Scler Int. 2017. 2017: 1-7
96. Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019. 92: e1029-40
97. Weinstock-Guttman B, Zivadinov R, Horakova D, Havrdova E, Qu J, Shyh G. Lipid profiles are associated with lesion formation over 24 months in interferon-β treated patients following the first demyelinating event. J Neurol Neurosurg Psychiatry. 2013. 84: 1186-91
98. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014. 29: 2520-6
99. Zarei S, Eggert J, Franqui-Dominguez L, Carl Y, Boria F, Stukova M. Comprehensive review of neuromyelitis optica and clinical characteristics of neuromyelitis optica patients in Puerto Rico. Surg Neurol Int. 2018. 9: 242-
100. Ziemssen T, Derfuss T, de Stefano N, Giovannoni G, Palavra F, Tomic D. Optimizing treatment success in multiple sclerosis. J Neurol. 2016. 263: 1053-65