- Department of Neurological Surgery, Vanderbilt University School of Medicine, Nashville, Tennessee, USA
- Vanderbilt Comprehensive Stroke Center, Vanderbilt University School of Medicine, Nashville, Tennessee, USA
- Department of Neurology, Vanderbilt University School of Medicine, Nashville, Tennessee, USA
- Department of Neurosurgery, Mt. Sinai School of Medicine, New York, USA
Correspondence Address:
Nishant Ganesh Kumar
Department of Neurosurgery, Mt. Sinai School of Medicine, New York, USA
DOI:10.4103/2152-7806.196366
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Scott L. Zuckerman, Jordan A. Magarik, Kiersten B. Espaillat, Nishant Ganesh Kumar, Ritwik Bhatia, Michael C. Dewan, Peter J. Morone, Lisa D. Hermann, Anne E. O’Duffy, Derek A. Riebau, Howard S. Kirshner, J. Mocco. Implementation of an institution-wide acute stroke algorithm: Improving stroke quality metrics. 21-Dec-2016;7:
How to cite this URL: Scott L. Zuckerman, Jordan A. Magarik, Kiersten B. Espaillat, Nishant Ganesh Kumar, Ritwik Bhatia, Michael C. Dewan, Peter J. Morone, Lisa D. Hermann, Anne E. O’Duffy, Derek A. Riebau, Howard S. Kirshner, J. Mocco. Implementation of an institution-wide acute stroke algorithm: Improving stroke quality metrics. 21-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/implementation-of-an-institution%e2%80%91wide-acute-stroke-algorithm-improving-stroke-quality-metrics/
Abstract
Background:In May 2012, an updated stroke algorithm was implemented at Vanderbilt University Medical Center. The current study objectives were to: (1) describe the process of implementing a new stroke algorithm and (2) compare pre- and post-algorithm quality improvement (QI) metrics, specificaly door to computed tomography time (DTCT), door to neurology time (DTN), and door to tPA administration time (DTT).
Methods:Our institutional stroke algorithm underwent extensive revision, with a focus on removing variability, streamlining care, and improving time delays. The updated stroke algorithm was implemented in May 2012. Three primary stroke QI metrics were evaluated over four separate 3-month time points, one pre- and three post-algorithm periods.
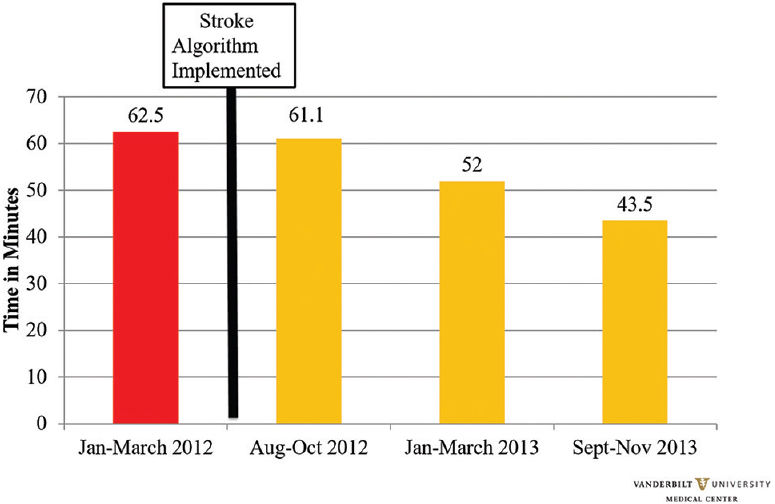
Results:The following data points improved after algorithm implementation: average DTCT decreased from 39.9 to 12.8 min (P P ≤ 0.001), and average DTT decreased from 62.5 to 43.5 min (P = 0.17).
Conclusion:A new stroke protocol that prioritized neurointervention at our institution resulted in significant lowering in the DTCT and DTN, with a nonsignificant improvement in DTT.
Keywords: Algorithm, emergency medicine, neurology, neurosurgery, stroke, thrombectomy
INTRODUCTION
Ischemic stroke is one of the leading causes of morbidity and mortality in the United States (US) resulting in approximately 795,000 first-time or recurrent strokes each year, of which 130,000 result in death.[
While these studies provide insight into developing a stroke algorithm, we hope to describe our institutional experience, focusing on the process involved. The Vanderbilt University Medical Center (VUMC) Departments of Neurology, Neurosurgery, Radiology, and Emergency Medicine revised an existing protocol for the preparation, response, and treatment of ischemic stroke patients. Prior to May 2012, a previous stroke algorithm was in effect. According to the old algorithm, a patient with acute, nontraumatic focal neurologic deficit concerning for ischemic stroke was identified in the ED or as an inpatient. A stroke alert page was triggered if the symptom onset was within 8 hours of last known normal and the stroke team went to the identified location, where the National Institute of Health Stroke Scale (NIHSS) was performed and the patient underwent a noncontrast head computed tomography scan (HCT). If no hemorrhage was seen, an update page was sent out based on NIHSS (NIHSS <6 involved the stroke neurology team only; NIHSS >6 also included the interventional team). In both scenarios, the focus was to identify potential IV tPA candidates and initiate infusion as soon as possible. With the evolution of interventional therapy, it was recognized that this protocol was not designed to efficiently identify interventional candidates. A detailed review of the protocol identified several areas of improvement.
In May 2012, the new and revised algorithm was implemented, such that every patient underwent the same neurological evaluation in every suspected ischemic stroke case. Herein, we report our institution's development and implementation of a hospital-wide acute stroke algorithm to minimize treatment delays and expedite care. The current study objectives were to (1) describe the new stroke algorithm and revision process in detail (2) compare pre- and post-algorithm quality improvement (QI) metrics.
MATERIALS AND METHODS
VUMC is a 584-bed tertiary care center designated by the Joint Commission as a Comprehensive Stroke Center[
Creating a new algorithm: Process of change
Three goals guided the revision process, namely (1) comparing the algorithm to the heavily protocol based field of trauma care, (2) eliminating excessive point-of-care decision making, and (3) removing time delays. Each point is expanded below.
Stroke trauma: The field of trauma is replete with evidence-based algorithms. Examples of this include transfusion practices,[ Eliminate excessive point-of-care decision making: The old algorithm contained several decision points. Eliminating such “on-the-fly” decisions would serve to minimize the influence of individual practitioner preferences. There would be no “if-then” choices; the same workup would ensue for every stroke patient. Furthermore, teams can work in parallel towards the next step once all parties are familiarized with the protocol. Remove time delays: By gathering multiple departments together, it was possible to identify where the time delays were and how care could be expedited. Three examples are noted below:
Paging system. In the prior system, when a patient was perceived to be a candidate for thrombectomy, the neurology team would have to determine which interventionalist was on call, manually page or call them, and wait for a response. In the new system, an updated stroke alert page went to all stakeholders, most importantly both the stroke neurologist and interventionalist. This allowed the stroke neurologist to focus on acute patient care while waiting for the interventionalist to call him/her directly. Decreasing antagonistic interactions. Frustration had been expressed that, while there had long been a goal for rapid CT, there were physician variations with ED staff. Some staff insisted the patient remain in the room until a full exam was performed, while others wanted them rushed to CT. After discussion, the group decided that the primary goal after the code stroke was to transport the patient immediately to the CT scanner. Moreover, if neurology arrived and the patient was already in the CT scanner, there would be discord between radiology and neurology. Neurology would want to rapidly obtain an exam for IV tPA evaluation, however, radiology would not want to occupy their scanner indefinitely for a clinical exam. This was resolved by recognizing the urgency of the plain CT, and then agreeing to allow a 5-minute pause while the patient was on the CT scanner and allow for a rapid neurological assessment to identify potential IV tPA candidates. Limit CTA/CTP technicians. VUMC previously rotated 36 CT technicians through all hospital scanners. This large volume of technicians resulted in limited exposure for each technician in performing the relatively specialized CTA and CTP scans. Limited technician experience created problems in the form of poor scan quality and inability to troubleshoot equipment problems. Though data does not exist to validate this change, halving the number of designated technicians theoretically increased experience, comfort level, speed, and study quality.
New algorithm
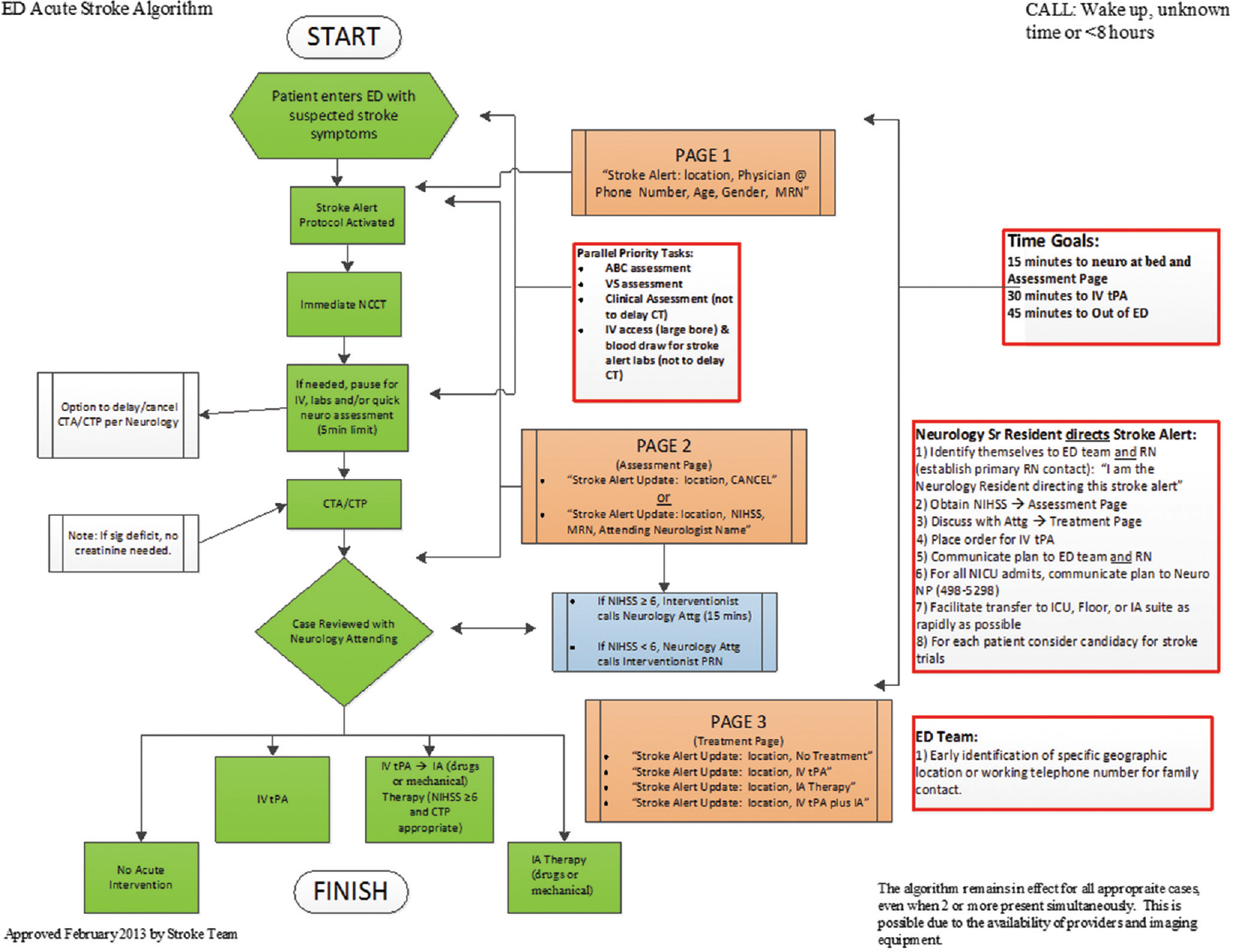
The current VUMC ED Stroke Algorithm is seen in
While the team is being alerted, the nurse or paramedic in the ED brings the patient directly to the CT scanner for an immediate HCT to assess acute hemorrhage. While the patient is being loaded onto the CT table, critical labs are drawn including glucose and an iStat Chem 8+ basic metabolic panel (BMP) + Creatinine. The iStat offers data on renal function within 2 minutes, and the HCT and CTA head/neck may be performed in succession. During a 5-minute pause for software-driven image reconstruction, the neurology resident may perform an NIHSS evaluation and exam. Next, the CTP commences while the neurology resident contacts the communications center to send out an “Assessment Page” that includes the NIHSS, the patient's medical record number, and the name of the attending stroke neurologist. The resident then calls the stroke attending to discuss the results of the HCT. If the NIHSS is a 5 or less, there is no automatic notification to the interventional team and the stroke attending decides if the patient is IV tPA eligible. Should the stroke attending desire, he or she may also contact the interventional attending for discussion and input. If the NIHSS is a 6 or higher, the interventional attending calls the stroke attending, while the stroke attending focuses on direct care. The interventionalist is first notified by the mass page that has been sent. IV tPA is often not delayed for interventionalist/stroke neurologist discussions or review of advanced imaging (CTA, CTP) and may be pursued in addition to the consideration for intervention. The stroke resident then sends out a final page with the treatment or cancellation decision.
If there is a decision to start IV tPA, the stroke resident places the order in the computer while simultaneously giving a verbal order to the nurse to expedite the acquisition and infusion of IV tPA. If the patient is an intervention candidate, with or without IV tPA, the patient is transported to the interventional suite.
The algorithm has several efficiency-promoting features, the most important of which include uniform, consistent, stepwise algorithm progression.
Study design
The updated stroke algorithm was fully implemented in May 2012. Data was collected from four different time points, all comprising three-month periods. The first time point was January–March 2012, pre-stroke algorithm implementation. Only 3 months of pre-stroke data was available due to incomplete paper records. The subsequent three time points were all after stroke algorithm implementation; August–October 2012, January–March 2013, and September–November 2013. These specific time points were chosen due to availability of records as complete documentation was not available during the initial phases of algorithm implementation. Study participants included any patient suspected of having an ischemic stroke, which activated the stroke alert system. Patients excluded from the study were those <18 years old. Institutional review board approval was not required as this study fell under the purview of quality improvement and did not meet the criteria for research (IRB #140895).
Statistical analysis
Three QI data points were collected, namely, door to CT time (DTCT), door to neurology evaluation time (DTN), and door to IV tPA administration time (DTT). Though additional variables exist to evaluate acute stroke care, these were the variables previously collected by the institution, which was kept constant. All time points were treated as continuous variables, measured in minutes. Each post-algorithm implementation time period (August–October 2012, January–March 2013, and September–November 2013) was compared to the pre-algorithm implementation time period (January–March 2012).
Parametric data was presented as mean ± standard deviation and compared via the Student's t-test. A P value of <0.05 was considered statistically significant. Any missing data was handled using the available case approach, thus only full cases with data were used. The sample size for each QI measure floated. All statistical analysis was performed in STATA version 14 (College Station, TX: StataCorp LP).
RESULTS
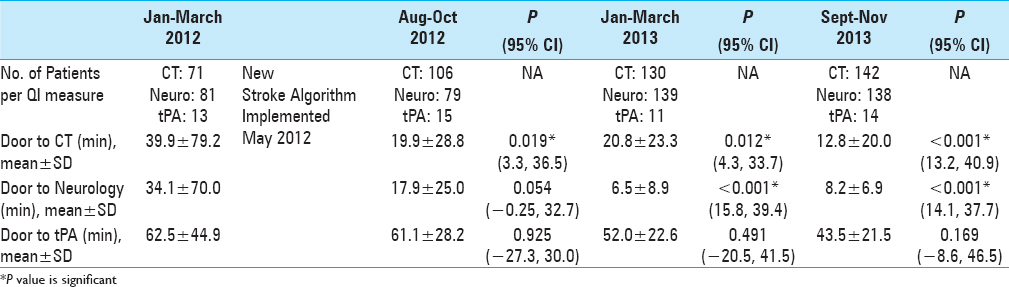
All QI data is summarized in
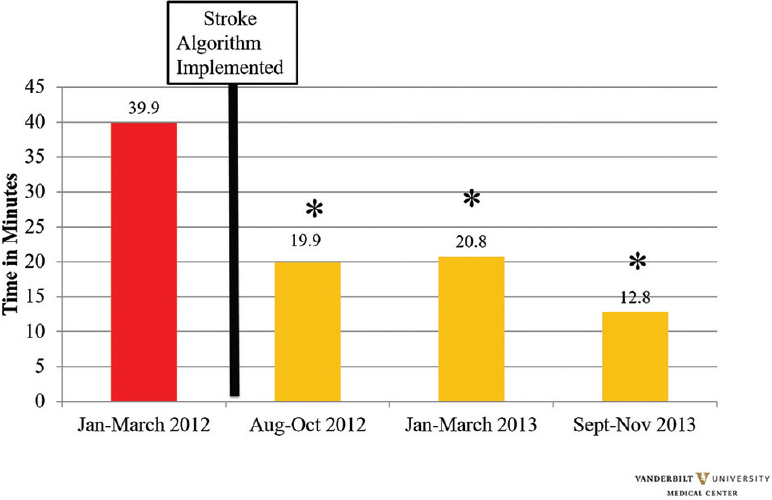
Door to computed tomography scan time (DTCT)
At each time point post-algorithm implementation, a significant decrease in DTCT was seen. Though a slight increase was seen from August–October 2012 (19.9 ± 28.8, P = 0.019) to January–March 2013 (20.8 ± 23.3, P = 0.012), this number dropped significantly in September–November 2013 (12.8 ± 20.0, P < 0.001). Each post-algorithm time period achieved statistical significance [
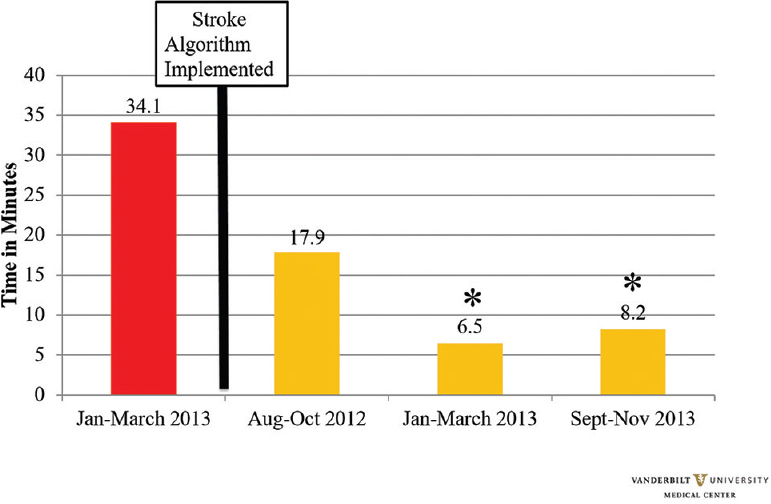
Door to neurology time (DTN)
At each time point post-algorithm implementation, except for the first data collection period, a significant decrease in DTN was seen. Though a non-statistically significant result was seen with a slight decrease in August–October 2012 (17.9 ± 25.0, P = 0.054), statistical significance was seen in the following 2 months where the time to neurology time plummeted in January–March 2013 (6.5 ± 8.9, P < 0.001) and September–November 2013 (8.2 ± 6.9, P < 0.001) [
Door to tPA time (DTT)
Despite significant findings seen in CT and neurology time, DTT did not achieve a level of statistical significance. The time to IV tPA dropped from 62.5 ± 44.9 min to 43.5 ± 21.5 minutes (P = 0.169). However, the goal time to DTT of 45 min was achieved [
DISCUSSION
Herein, we describe the revision and implementation of an acute stroke algorithm and report pre- and post-algorithm quality metrics. The intervention was a joint effort between multiple departments, physicians, nurses, and hospital staff to provide a unified, consistent method of assessment and treatment of suspected ischemic stroke patients. After algorithm implementation, a statistically significant decrease in several key quality metrics was observed.
In ischemic stroke, the correlation between earlier revascularization and improved outcomes has been well established.[
Several institutional-wide stroke algorithms have gained traction in improving stroke outcome metrics.[
Several other QI interventions have been linked to improved time to treatment such as pre-notification by EMS,[
Interestingly, while our changes resulted in significant reductions in DTCT and DTN evaluation, there was a non-significant reduction in the DTT. Potential reasons for the non-significant reduction include pre-hospital delays and in-hospital delays, as outlined by Desai et al.[
Overall, the ischemic stroke community has seen an expansion in initiatives to expedite acute stroke care. Efforts have been made to improve time to imaging, neurologic evaluation, and intravenous or intra-arterial treatment. We have aimed to describe our institutional experience in detail for the benefit of other institutions. Only through successful collaboration, across departments, physicians, nurses, and hospital staff, can systems be improved and care of the acute stroke care be expedited.
The current study is not without limitation. First, this is a retrospective study of a prospectively maintained database. Because of the quality improvement nature of our study, we failed to collect demographic data of patients. Thus, race, gender, and age could not be accounted for in the statistical analysis. In addition, due to gaps in data collection, QI data was not available for all pre- and post-intervention months. Third, while we demonstrated improved quality metrics at the initial stroke evaluation, we did not collect long-term clinical outcomes including modified Rankin scores to determine what effect, if any, these improved metrics had on clinical outcome. Future direction of the study will be to include clinical outcomes, as well as include analysis of interventional therapies as these are an important adjunct to intravenous thrombolytics, are time sensitive, and subject to delays in initial acute stroke triage.
CONCLUSION
An institutional-wide acute stroke algorithm was successfully designed and implemented through a multi-disciplinary approach. After revision and implementation, significant lowering in the time for CT scan and neurology evaluation, and a non-significant improvement in time to IV tPA administration was seen. Future studies should focus on correlating individual patient factors with time parameters, in addition to long-term patient outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
The study was approved by Institutional Review Board (#140895).
References
1. Ahmed N, Whelan J, Brownlee J, Chari V, Chung R. The contribution of laparoscopy in evaluation of penetrating abdominal wounds. J Am Coll Surg. 2005. 201: 213-6
2. Aoun RJ, Bendok BR, Zammar SG, Hamade YJ, Aguilar MI, Demaerschalk BM. From Delivering the Patient to the Hospital to Delivering the Hospital to the Patient: Acute Stroke Therapy in an Ambulance. World Neurosurg. 2015. 84: 204-5
3. Bathala L. A visit to the stroke belt of the United States. J Neurosci Rural Pract. 2012. 3: 426-8
4. Benavente L, Villanueva MJ, Vega P, Casado I, Vidal JA, Castano B. Code stroke in Asturias. Neurologia. 2016. 31: 143-8
5. Biffl WL, Kaups KL, Pham TN, Rowell SE, Jurkovich GJ, Burlew CC. Validating the Western Trauma Association algorithm for managing patients with anterior abdominal stab wounds: A Western Trauma Association multicenter trial. Journal Trauma. 2011. 71: 1494-502
6. Cherry RA, Eachempati SR, Hydo LJ, Barie PS. The role of laparoscopy in penetrating abdominal stab wounds. Surg Laparosc Endosc Percutan Tech. 2005. 15: 14-7
7. Collier B, Diaz J, Forbes R, Morris J, May A, Guy J. The impact of a normoglycemic management protocol on clinical outcomes in the trauma intensive care unit. JPEN J Parenter Enteral Nutr. 2005. 29: 353-8
8. Desai JA, Smith EE. Prenotification and other factors involved in rapid tPA administration. Curr Atheroscler Rep. 2013. 15: 337-
9. Dortch MJ, Mowery NT, Ozdas A, Dossett L, Cao H, Collier B. A computerized insulin infusion titration protocol improves glucose control with less hypoglycemia compared to a manual titration protocol in a trauma intensive care unit. JPEN J Parenter Enteral Nutr. 2008. 32: 18-27
10. Ebinger M, Winter B, Wendt M, Weber JE, Waldschmidt C, Rozanski M. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: A randomized clinical trial. JAMA. 2014. 311: 1622-31
11. Fakhraldeen M, Segal E, de Champlain F. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: A randomized clinical trial. CJEM. 2015. 17: 709-12
12. Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: Patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011. 123: 750-8
13. Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED. Improving door-to-needle times in acute ischemic stroke: The design and rationale for the American Heart Association/American Stroke Association's Target: Stroke initiative. Stroke. 2011. 42: 2983-9
14. Fonarow GC, Zhao X, Smith EE, Saver JL, Reeves MJ, Bhatt DL. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014. 311: 1632-40
15. Gonzalez RG, Copen WA, Schaefer PW, Lev MH, Pomerantz SR, Rapalino O. The Massachusetts General Hospital acute stroke imaging algorithm: An experience and evidence based approach. J Neurointerv Surg. 2013. 5: i7-12
16. Govindarajan P, Ghilarducci D, McCulloch C, Pierog J, Bloom E, Johnston C. Comparative evaluation of stroke triage algorithms for emergency medical dispatchers (MeDS): Prospective cohort study protocol. BMC Neurol. 2011. 11: 14-
17. Gropen TI, Gagliano PJ, Blake CA, Sacco RL, Kwiatkowski T, Richmond NJ. Quality improvement in acute stroke: The New York State Stroke Center Designation Project. Neurology. 2006. 67: 88-93
18. Grotta JC, Burgin WS, El-Mitwalli A, Long M, Campbell M, Morgenstern LB. Intravenous tissue-type plasminogen activator therapy for ischemic stroke: Houston experience 1996 to 2000. Arch Neurol. 2001. 58: 2009-13
19. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995. 274: 1017-25
20. Hendrickson JE, Shaz BH, Pereira G, Parker PM, Jessup P, Atwell F. Implementation of a pediatric trauma massive transfusion protocol: One institution's experience. Transfusion. 2012. 52: 1228-36
21. Heo JH, Kim YD, Nam HS, Hong KS, Ahn SH, Cho HJ. A computerized in-hospital alert system for thrombolysis in acute stroke. Stroke. 2010. 41: 1978-83
22. Hill LVU Stroke Center Attains New Level of Certification. 2013. p.
23. Hoegerl C, Goldstein FJ, Sartorius J. Implementation of a stroke alert protocol in the emergency department: A pilot study. J Am Osteopath Assoc. 2011. 111: 21-7
24. Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013. 44: 870-947
25. Jeerakathil T, Shuaib A, Majumdar SR, Demchuk AM, Butcher KS, Watson TJ. The Alberta Stroke Prevention in TIAs and mild strokes (ASPIRE) intervention: Rationale and design for evaluating the implementation of a province-wide TIA triaging system. Int J Stroke. 2014. 9: 135-43
26. Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009. 73: 1066-72
27. Kutcher ME, Pepper MB, Morabito D, Sunjaya D, Knudson MM, Cohen MJ. Finding the sweet spot: Identification of optimal glucose levels in critically injured patients. J Trauma. 2011. 71: 1108-14
28. Liebenberg WA, Demetriades AK, Hankins M, Hardwidge C, Hartzenberg BH. Penetrating civilian craniocerebral gunshot wounds: A protocol of delayed surgery. Neurosurgery. 2005. 57: 293-9
29. Lin CB, Peterson ED, Smith EE, Saver JL, Liang L, Xian Y. Emergency medical service hospital prenotification is associated with improved evaluation and treatment of acute ischemic stroke. Circ Cardiovasc Qual Outcomes. 2012. 5: 514-22
30. Lindsberg PJ, Happola O, Kallela M, Valanne L, Kuisma M, Kaste M. Door to thrombolysis: ER reorganization and reduced delays to acute stroke treatment. Neurology. 2006. 67: 334-6
31. McKinney JS, Mylavarapu K, Lane J, Roberts V, Ohman-Strickland P, Merlin MA. Hospital prenotification of stroke patients by emergency medical services improves stroke time targets. J Stroke Cerebrovasc Dis. 2013. 22: 113-8
32. Nam HS, Han SW, Ahn SH, Lee JY, Choi HY, Park IC. Improved time intervals by implementation of computerized physician order entry-based stroke team approach. Cerebrovasc Dis. 2007. 23: 289-93
33. Nolte CH, Malzahn U, Kuhnle Y, Ploner CJ, Muller-Nordhorn J, Mockel M. Improvement of door-to-imaging time in acute stroke patients by implementation of an all-points alarm. J Stroke Cerebrovasc Dis. 2013. 22: 149-53
34. Nunez TC, Young PP, Holcomb JB, Cotton BA. Creation, implementation, and maturation of a massive transfusion protocol for the exsanguinating trauma patient. J Trauma. 2010. 68: 1498-505
35. Prevention CfDCa. Cerebrovascular disease or stroke. 2013. p.
36. Prevention CfDCa. First. ever county level report on stroke hospitalizations. CDC Press. 2006. p.
37. Prevention CfDCa. Stroke Facts. 2013. p.
38. Prevention. CfDCa. Atlas of heart disease and stroke. 2012. p.
39. Qureshi AI, Kirmani JF, Sayed MA, Safdar A, Ahmed S, Ferguson R. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology. 2005. 64: 2115-20
40. Richards JE, Kauffmann RM, Zuckerman SL, Obremskey WT, May AK. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J Bone Joint Surg Am. 2012. 94: 1181-6
41. Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM. Massive transfusion protocols: The role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009. 209: 198-205
42. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM. American Heart Association Statistical Update: Heart Disease and Stroke Statistics – 2011 Update. Circulation. 2011. 123: e18-209
43. Rose KM, Rosamond WD, Huston SL, Murphy CV, Tegeler CH. Predictors of time from hospital arrival to initial brain-imaging among suspected stroke patients: The North Carolina Collaborative Stroke Registry. Stroke. 2008. 39: 3262-7
44. Sattin JA, Olson SE, Liu L, Raman R, Lyden PD. An expedited code stroke protocol is feasible and safe. Stroke. 2006. 37: 2935-9
45. Sauser K, Levine DA, Nickles AV, Reeves MJ. Hospital variation in thrombolysis times among patients with acute ischemic stroke: The contributions of door-to-imaging time and imaging-to-needle time. JAMA Neurol. 2014. 71: 1155-61
46. Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013. 309: 2480-8
47. Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: Comparison with CT in hyperacute intracerebral hemorrhage. Stroke. 1999. 30: 765-8
48. Schrock JW, Lum M. Drill down analysis of door-to-needle time of acute ischemic stroke patients treated with intravenous tissue plasminogen activator. Am J Emerg Med. 2014. 32: 1330-3
49. Spiotta AM, Vargas J, Turner R, Chaudry MI, Battenhouse H, Turk AS. The golden hour of stroke intervention: Effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointervent Surg. 2014. 6: 511-6
50. Sumislawski JJ, Zarzaur BL, Paulus EM, Sharpe JP, Savage SA, Nawaf CB. Diagnostic laparoscopy after anterior abdominal stab wounds: Worth another look?. J Trauma Acute Care Surg. 2013. 75: 1013-7
51. Sung SF, Huang YC, Ong CT, Chen W. Validity of a computerised five-level emergency triage system for patients with acute ischaemic stroke. Emerg Med J. 2013. 30: 454-8
52. Sung SF, Ong CT, Wu CS, Hsu YC, Su YH. Increased use of thrombolytic therapy and shortening of in-hospital delays following acute ischemic stroke: Experience on the establishment of a primary stroke center at a community hospital. Acta Neurol. 2010. 19: 246-52
53. Tai YJ, Weir L, Hand P, Davis S, Yan B. Does a ‘code stroke’ rapid access protocol decrease door-to-needle time for thrombolysis?. Intern Med J. 2012. 42: 1316-24
54. Tong D, Reeves MJ, Hernandez AF, Zhao X, Olson DM, Fonarow GC. Times from symptom onset to hospital arrival in the Get with the Guidelines--Stroke Program 2002 to 2009: Temporal trends and implications. Stroke. 2012. 43: 1912-7
55. Van Schaik SM, Scott S, de Lau LM, Van den Berg-Vos RM, Kruyt ND. Short Door-to-Needle Times in Acute Ischemic Stroke and Prospective Identification of Its Delaying Factors. Cerebrovasc Dis Extra. 2015. 5: 75-83
56. Vora N, Tung CE, Mlynash M, Garcia M, Kemp S, Kleinman J. TIA triage in emergency department using acute MRI (TIA-TEAM): A feasibility and safety study. Int J Stroke. 2015. 10: 343-7
57. Walter S, Kostopoulos P, Haass A, Keller I, Lesmeister M, Schlechtriemen T. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: A randomised controlled trial. Lancet Neurol. 2012. 11: 397-404
58. Walter S, Kostopoulos P, Haass A, Lesmeister M, Grasu M, Grunwald I. Point-of-care laboratory halves door-to-therapy-decision time in acute stroke. Ann Neurol. 2011. 69: 581-6
59. Weber JE, Ebinger M, Rozanski M, Waldschmidt C, Wendt M, Winter B. Prehospital thrombolysis in acute stroke: Results of the PHANTOM-S pilot study. Neurology. 2013. 80: 163-8
60. Wendt M, Ebinger M, Kunz A, Rozanski M, Waldschmidt C, Weber JE. Improved prehospital triage of patients with stroke in a specialized stroke ambulance: Results of the pre-hospital acute neurological therapy and optimization of medical care in stroke study. Stroke. 2015. 46: 740-5