- Department of Neurosurgery, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, United States
- Department of Neurosurgery, Boston Children’s Hospital, Boston, Massachusetts, United States.
Correspondence Address:
Sudhakar Vadivelu
Department of Neurosurgery, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, United States
DOI:10.25259/SNI_156_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zachary Porter, George Yang, Shawn Vuong, Baher Hanna, Joseph Madsen, Sudhakar Vadivelu. In situ clearance of a proximal shunt malfunction in a child with hydrocephalus post cerebral arteriovenous malformation rupture noted intraoperatively. 16-May-2020;11:116
How to cite this URL: Zachary Porter, George Yang, Shawn Vuong, Baher Hanna, Joseph Madsen, Sudhakar Vadivelu. In situ clearance of a proximal shunt malfunction in a child with hydrocephalus post cerebral arteriovenous malformation rupture noted intraoperatively. 16-May-2020;11:116. Available from: https://surgicalneurologyint.com/surgicalint-articles/10032/
Abstract
Background: Hydrocephalus shunt malfunctions remain treated with surgical intervention only. Despite efforts at identifying or preventing CSF shunt obstruction, no evidence currently exists to restore CSF flow following proximal occlusion, non-invasively.
Case Description: We present direct intraoperative evidence in the case of a 5-year-old male who developed hydrocephalus subsequent to hemorrhagic presentation post cerebral arteriovenous malformation rupture. After weeks of externalized CSF diversion for clearance of CSF red blood cells, he was taken to the operating room for removal of the external ventricular drain and placement of a ventriculoperitoneal shunt for hydrocephalus. At conclusion of placing his ventriculoperitoneal shunt with ReFlow flusher assist device, his shunt valve reservoir was noted to not refill. Following manual depression of the ReFlow flusher, we identified clearance of debris from the obstructed ventricular catheter allowing reestablished CSF flow through the shunt system under live intraoperative ultrasonography. Subsequently, there was return of brisk refill to the shunt valve reservoir.
Conclusion: Observations here demonstrate a potentially useful technical strategy toward clearance of proximal shunt obstructions, in situ.
Keywords: Posthemorrhagic hydrocephalus, Intraventricular hemorrhage
INTRODUCTION
Nearly half of all CSF shunt failures result from obstruction of the proximal ventricular catheter.[
Here, we present a novel intraoperative ultrasonographic observation of new shunt technology that utilized a non- invasive mechanism to clear an obstructed proximal ventricular catheter, in situ.
Technical case report
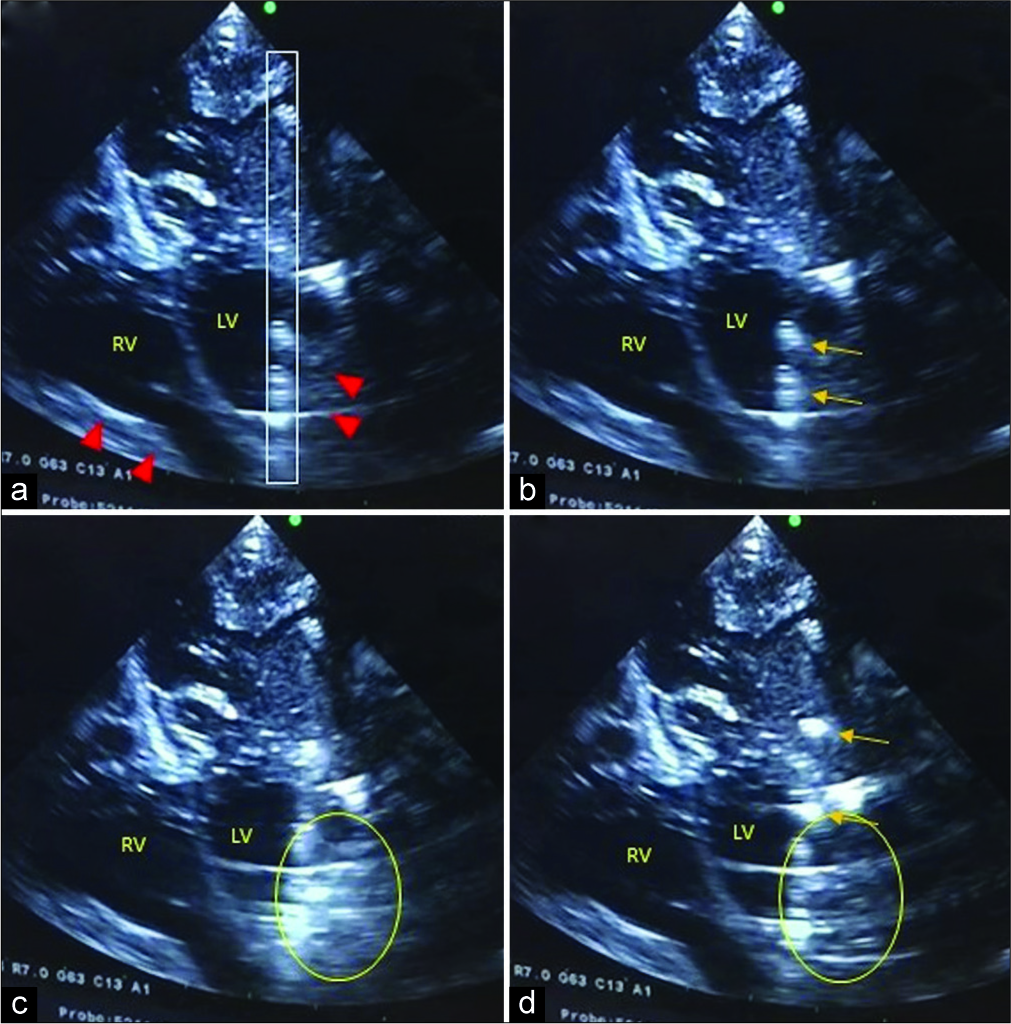
The patient is a 5-year-old male who developed hydrocephalus following an intraventricular hemorrhage secondary to pial AVM rupture. After endovascular embolization stabilization of the AVM and intraventricular hemorrhage controlled with temporary external ventricular drainage, he subsequently required ventriculoperitoneal shunt placement. At the time of internalization, his CSF profile included a protein count of 23 and RBC count of <50. We discussed with the family, consent for the placement of a novel shunt design that includes a ReFlow proximal catheter with relief membrane and assistive flusher device (Anuncia, Lowell MA) that allows for retrograde proximal catheter tip flushing for relief of debris occluding the proximal catheter tip in the ventricle through an assistive reservoir device placed in line before the shunt valve; this shunt construct allows for external depression of the assist device reservoir to induce retrograde flush to the proximal catheter tip to reestablish anterograde CSF flow through the ventricular catheter, in situ. The patient was brought to operating room where we first removed the external ventricular drain and then proceeded to tunnel the Codman Bactiseal distal catheter to the peritoneal space first and the Codman Certas valve attached proximally to the distal catheter and residing in the subgaleal cranial pocket. We then under live ultrasonography placed the ReFlow ventricular catheter into the frontal horn of the left lateral ventricle and away from the remaining intraventricular hemorrhage along the lateral wall [
Figure 1:
(a) Intraoperative ultrasonography in burr hole probe’s eye view demonstrating bilateral residual intraventricular hemorrhage (red arrowheads) along the lateral walls of the bilateral frontal ventricular horns with the ReFlow ventricular catheter (gray rectangle) within the left lateral ventricle (LV). (b) Lack of flow within the ventricular catheter with echoic densities (orange arrows). (c) Immediate single image post ReFlow flusher depressed, demonstrating extraluminal echogenic debris dispersing from the adjacent catheter inlet holes (yellow oval). (d) Ventricular clearance extraluminally (yellow oval) after few seconds and intraluminal CSF flow restored in anterograde flow (orange arrows).
After complete placement of his shunt system both ventricular and peritoneal sites and immediately prior to skin closure, his Certas valve was noted to not refill. With this concern, we manually depressed the ReFlow flusher device to generate a retrograde flush of CSF from the ReFlow CSF chamber to the ReFlow proximal catheter tip [
DISCUSSION
This is the first (ultrasonographic) evidence of a novel strategy to clear proximal shunt occlusions non-invasively, which would, otherwise, require invasive surgical revision.[
We could not identify here that this relief membrane deployed or that it played a role in the re-establishment of ventricular catheter CSF anterograde flow. It is possible, however, that retrograde CSF flow established from manual depression of the ReFlow flusher device was sufficient to reestablish anterograde flow through the ventricular catheter without the needing to break the relief membrane. Second, great care was taken to document the ReFlow pump and programmable valve positioning extracranially for use during future shunt evaluations. In addition, we took great effort to note, intracranially, location of the catheter tip as it is not clear whether the catheter tip relief membrane requires adequate ventricular CSF volume surrounding it to be effective. Third, we could not quantify the amount of debris that was successfully cleared during this observation and longevity of this mechanism. Thus, the extent as to how much debris can be freed and the longevity of successful clearing without the need of replacement of the shunt system is beyond the scope of our observation reported in this report. Fourth, the observations reported here cannot be ascribed to clinical benefit given that the obstruction occurred in the OR while the patient remained under general anesthesia.
The observations reported here support a novel strategy toward the potential treatment of shunt malfunctions due to proximal occlusions. It remains to be tested whether this novel approach truly represents a mechanically viable option for nonsurgical treatment of proximal shunt obstruction outside of the operative theater. Long-term follow-up is needed in our patient to determine clinical benefit and cost- effectiveness of implantation of this new technical approach.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare an interest in materials presented herein as follows: Dr. Joseph R. Madsen is a Co-Founder of Anuncia and Co-Inventor of the ReFlow Ventricular System. Dr. Sudhakar Vadivelu is a consultant for Alycone lifesciences.
Acknowledgements
The authors thank Dr. Brian Coley for his expert technical guidance regarding CSF flow and catheter depiction under ultrasonography and Ms. Sue Mastruserio with manuscript editing.
References
1. Bierbrauer KS, Storrs BB, McLone DG, Tomita T, Dauser R. A prospective, randomized study of shunt function and infections as a function of shunt placement. Pediatr Neurosurg. 1990. 16: 287-91
2. Browd SR, Ragel BT, Gottfried ON, Kestle JR. Failure of cerebrospinal fluid shunts: Part I: Obstruction and mechanical failure. Pediatr Neurol. 2006. 34: 83-92
3. Chen Q, Feng Z, Tan Q, Guo J, Tang J, Tan L. Post-hemorrhagic hydrocephalus: Recent advances and new therapeutic insights. J Neurol Sci. 2017. 375: 220-30
4. Di Rocco C, Marchese E, Velardi F. A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus. Cooperative survey of the 1991-1992 education committee of the ISPN. Childs Nerv Syst. 1994. 10: 321-7
5. Drake JM, Kestle JRW, Milner R, Cinalli G, Boop F, Piatt J. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998. 43: 294-303
6. Flannery AM, Duhaime AC, Tamber MS, Kemp J. Pediatric hydrocephalus: Systematic literature review and evidence-based guidelines. Part 3: Endoscopic computer-assisted electromagnetic navigation and ultrasonography as technical adjuvants for shunt placement. J Neurosurg Pediatr. 2014. 14: 24-9
7. Flitter MA, Buchheit WA, Murtagh F, Lapayowker MS. Ultrasound determination of cerebrospinal fluid shunt patency. Technical note. J Neurosurg. 1975. 42: 728-30
8. Fulkerson DH, Vachhrajani S, Bohnstedt BN, Patel NB, Patel AJ, Fox BD. Analysis of the risk of shunt failure or infection related to cerebrospinal fluid cell count, protein level, and glucose levels in low-birth-weight premature infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 2011. 7: 147-51
9. Hartman R, Aglyamov S, Fox DJ, Emelianov S. Quantitative contrast-enhanced ultrasound measurement of cerebrospinal fluid flow for the diagnosis of ventricular shunt malfunction. J Neurosurg. 2015. 123: 1420-6
10. Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F. Long-term follow-up data from the shunt design trial. Pediatr Neurosurg. 2000. 33: 230-6
11. Kulkarni A V, Riva-Cambrin J, Butler J, Browd SR, Drake JM, Holubkov R. Outcomes of CSF shunting in children: Comparison of hydrocephalus clinical Research network cohort with historical controls: Clinical article. J Neurosurg Pediatr. 2013. 12: 334-8
12. Lazareff JA, Peacock W, Holly L, Ver Halen J, Wong A, Olmstead C. Multiple shunt failures: An analysis of relevant factors. Child’s Nerv Syst. 1998. 14: 271-5
13. Mazzola CA, Choudhri AF, Auguste KI, Limbrick D, Rogido M, Mitchell L. Pediatric hydrocephalus: Systematic literature review and evidence-based guidelines. Part 2: Management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr. 2014. 14: 8-23
14. Riva-Cambrin J, Kestle JRW, Holubkov R, Butler J, Kulkarni A V, Drake J. Risk factors for shunt malfunction in pediatric hydrocephalus: A multicenter prospective cohort study. J Neurosurg Pediatr. 2016. 17: 382-90
15. Sainte-Rose C, Piatt JH, Renier D, Pierre-Kahn A, Hirsch JF, Hoffman HJ. Mechanical complications in shunts. Pediatr Neurosurg. 1991. 17: 2-9