- Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama, United States.
Correspondence Address:
Borna Ethan Tabibian, Department of Neurosurgery, University of Alabama at Birmingham, Birmingham, Alabama, United States.
DOI:10.25259/SNI_183_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Borna Ethan Tabibian, Elizabeth Liptrap, Jesse Jones. Incidentally discovered dural arteriovenous fistula during middle meningeal artery embolization for the treatment of chronic subdural hematoma. 30-Aug-2021;12:438
How to cite this URL: Borna Ethan Tabibian, Elizabeth Liptrap, Jesse Jones. Incidentally discovered dural arteriovenous fistula during middle meningeal artery embolization for the treatment of chronic subdural hematoma. 30-Aug-2021;12:438. Available from: https://surgicalneurologyint.com/surgicalint-articles/11071/
Abstract
Background: The incidence of chronic subdural hematoma (CSDH) is increasing with population age and anticoagulant use. Embolization of the middle meningeal artery (MMA) is an emerging, less invasive alternative to open surgery in treating this condition. Dural arteriovenous fistula (DAVF) is a rare condition whose association with CSDH is not well understood. We present three cases with incidentally discovered DAVFs during MMA embolization for the treatment of CSDH that necessitated adjustments to initial treatment strategy.
Case Descriptions: We retrospectively reviewed all MMA embolizations performed for the treatment of CSDH beginning in 9/2019 to 11/2020. Imaging and hospital course of three cases of incidentally discovered DAVF, including patient demographics, clinical presentation, methods of treatment, imaging and outcome were assessed. Thirty MMA embolizations were performed as primary or adjunct treatment of CSDH. DAVF was discovered angiographically in 3 (10%) cases. All patients reported a history of prior closed head injury, although the timing of injury and subdural blood product age did not correlate in 2 of the 3 cases. All subjects experienced complete symptomatic and radiographic resolution of the subdural hematoma and DAVF following intervention.
Conclusion: As MMA embolization for CSDH becomes more frequent, so may the incidental diagnosis of DAVF. Awareness of this potential association is critical to diagnosing DAVF with angiography and altering treatment strategies as needed.
Keywords: Dural arteriovenous fistula, Embolization, Endovascular, Middle meningeal artery, Subdural hematoma
INTRODUCTION
Chronic subdural hematoma (CSDH) is a common disease among elderly population, and the prevalence continues to rise with trends toward increased life expectancy of the general population.[
To date, no study has addressed the association between DAVF and CSDH; with increased adoption of MMA embolization, however, this topic now warrants further investigation and awareness. We present three cases in which DAVF was discovered incidentally during MMA embolization for CSDH and required adjustments to the treatment plan.
MATERIALS AND METHODS
Among 65 patients evaluated in consultation or admitted to the neurosurgery service from September 2019 to November 2020, we performed 30 MMA embolizations (46%) as primary or adjunct treatment for CSDH. All cases were performed at a single institution by a fellowship-trained neurointerventionalist (JGAJ). Among all cases, 3 (10%) were found to harbor ipsilateral DAVF on angiography. The patient specific characteristics, presenting symptoms, hospital course, procedural details, operative reports, images, and outcomes were reviewed. IRB approval was obtained based on institutional protocol.
ILLUSTRATIVE CASES
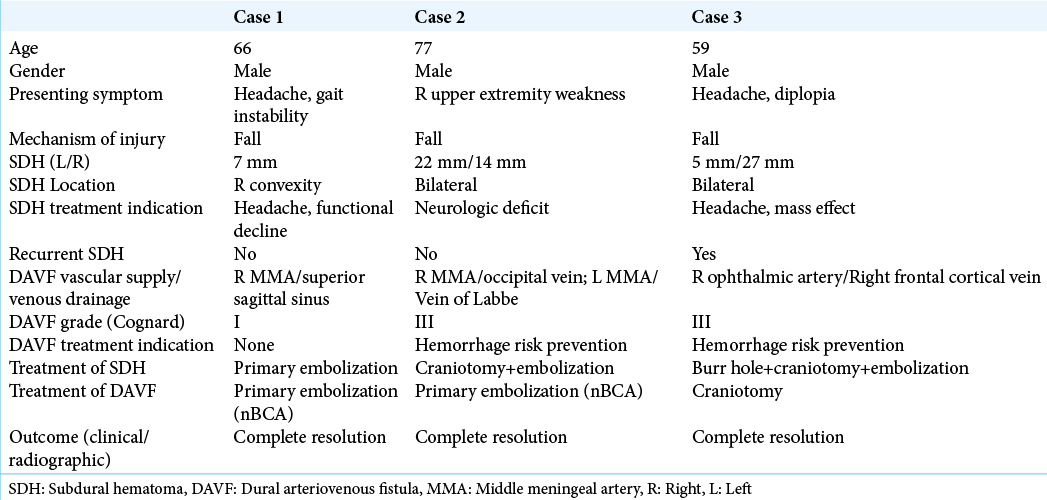
Case 1
A 66-year-old male with a recent syncope-related fall and closed head injury presented with progressively worsening gait instability, dizziness, and headaches. The fall occurred 1 week before presentation. On examination, no focal neurologic deficits were found. Noncontrast head CT demonstrated a 7 mm right convexity mixed density subdural hematoma [
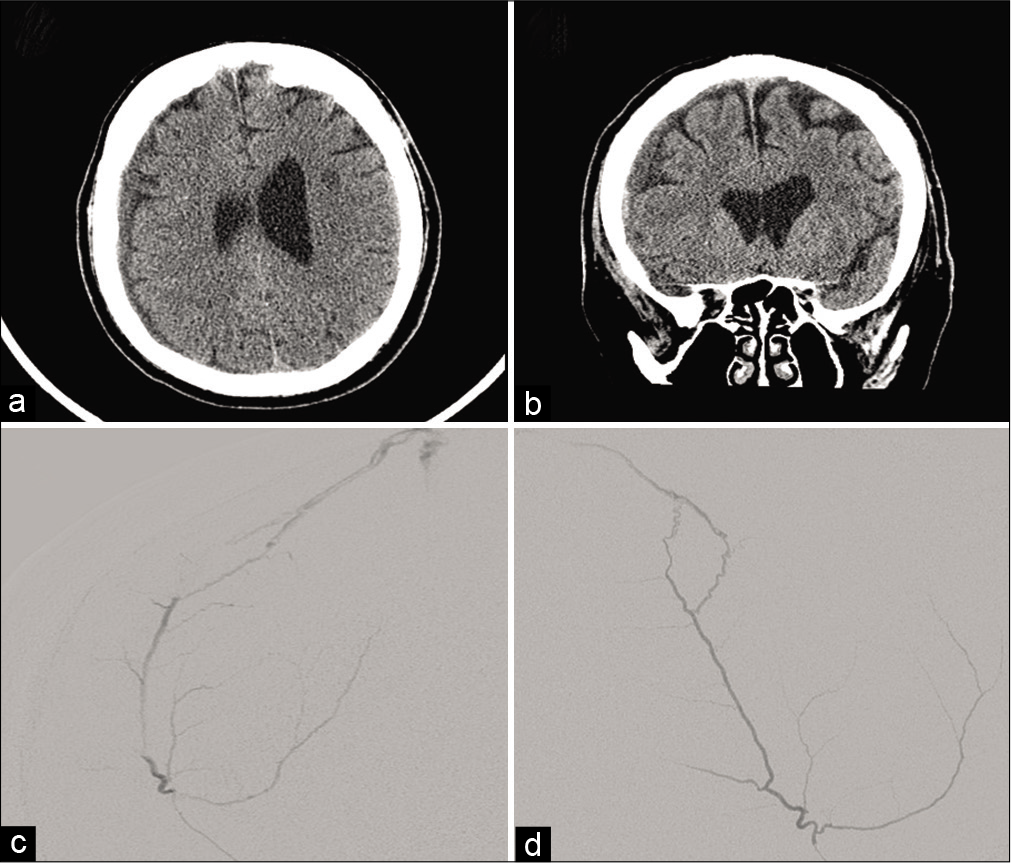
Figure 1:
(a and b) Axial and coronal noncontrast CT demonstrates a 7 mm right convexity mixed density subdural hematoma. (c and d) Anteroposterior and lateral projections, superselective catheter angiogram, and right middle meningeal artery frontoparietal branch injection demonstrating a right parietal dural arteriovenous fistula draining into the superior sagittal sinus.
Case 2
A 77-year-old male with a recent syncope-related fall and closed head injury presented with gradually worsening isolated right upper extremity monoparesis. The fall occurred 1 month before presentation. On examination, the patient demonstrated 4/5 strength in his right upper extremity, with no additional neurologic deficits. Noncontrast head CT demonstrated bilateral mixed density convexity subdural hematomas [22 mm on the left and 14 mm on the right;
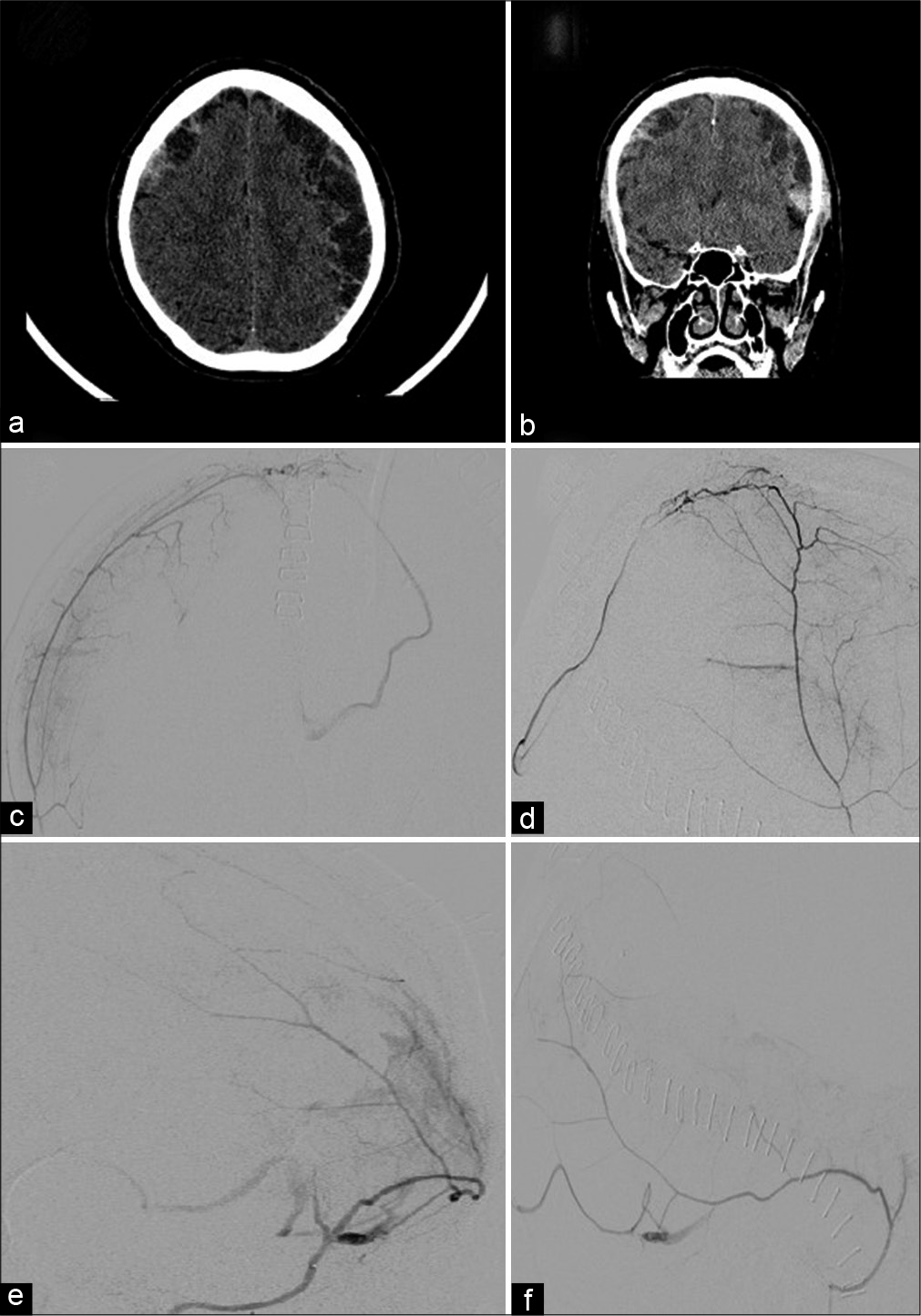
Figure 2:
(a and b) Axial and coronal noncontrast CT demonstrates bilateral mixed density convexity subdural hematomas. (c and d) Anteroposterior and lateral views, superselective catheter angiogram, and right middle meningeal artery parietal branch injection demonstrating a dural arteriovenous fistula (DAVF) draining into an occipital cortical vein. (e and f) Oblique anteroposterior and lateral views, superselective catheter angiogram, and left middle meningeal artery temporal branch injection demonstrating a DAVF draining into vein of Labbe.
Case 3
A 59-year-old male with a recent fall and closed head injury presented with progressively worsening headaches. The fall occurred 9 days before presentation. On examination, no neurologic deficits were found. Noncontrast head CT demonstrated a 5 mm left convexity CSDH [
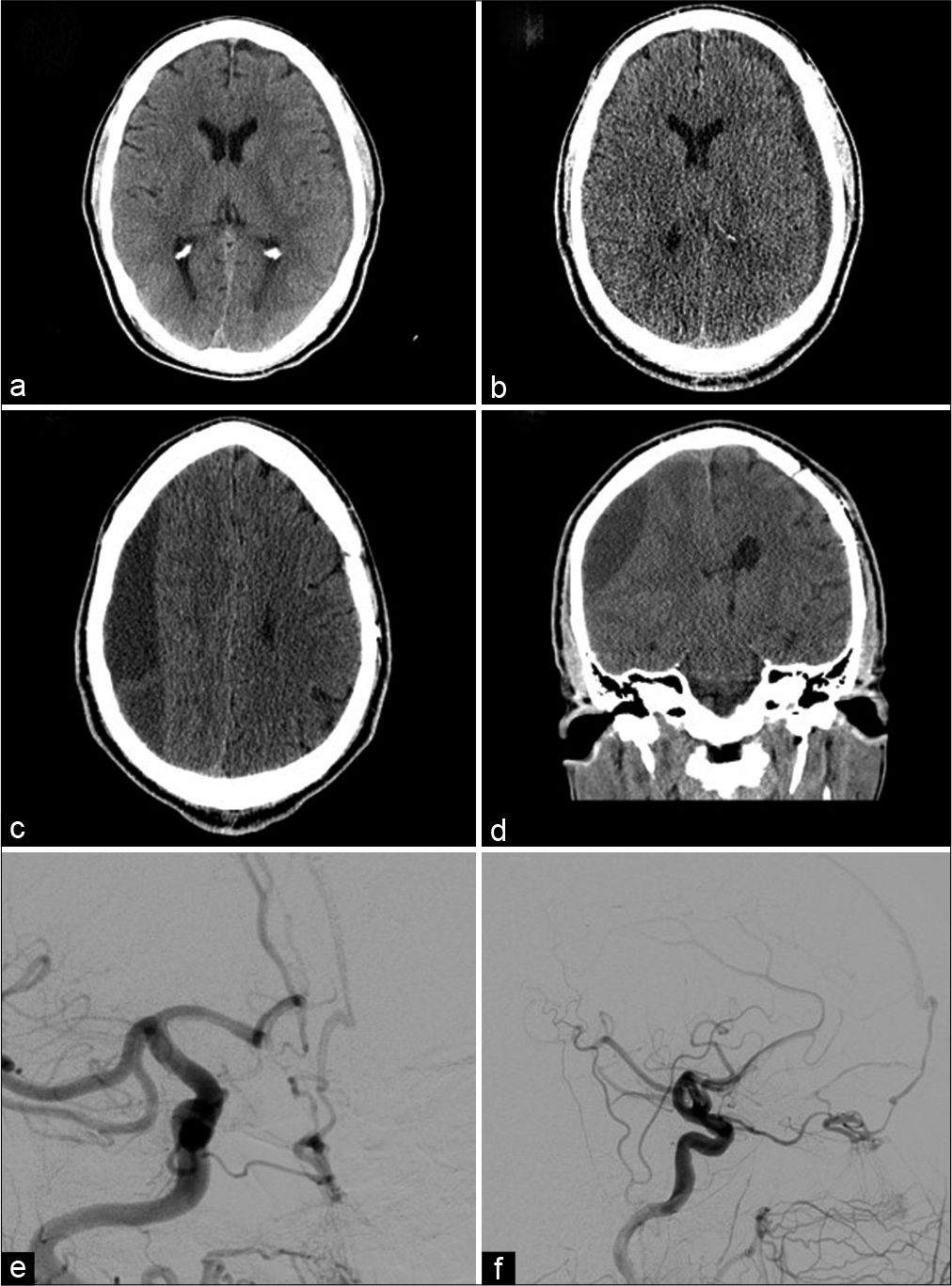
Figure 3:
(a) Axial noncontrast CT demonstrates 5 mm left convexity chronic subdural hematoma corresponding to the patient’s initial presentation. (b) Axial noncontrast CT demonstrates interval increase in size of the left convexity subdural hematoma. (c and d) Axial and coronal noncontrast CT performed 1 month later demonstrates a new large right subacute on chronic subdural hematoma. (e and f) Anteroposterior and lateral views, selective catheter angiogram, and right common carotid artery injection demonstrating a cribriform plate dural arteriovenous fistula with ophthalmic artery feeders draining into a right frontal cortical vein.
DISCUSSION
Although it may often be assumed that an initial trauma incites both DAVF and CSDH, as in the patients described herein, the causal relationship between these entities remains unclear. Patients 1 and 3 endorsed recent head trauma but their subdural hematoma appeared chronic; patient 3 developed a new contralateral CSDH without intervening head trauma in the setting of a known DAVF. Nevertheless, this report underscores the importance of angiographically assessing for DAVF before MMA embolization. DAVF is often identified during angiography performed after recent head trauma.[
Further, investigation into whether CSDH predisposes to DAVF, or vice versa, represents an important research topic that has potential to increase our understanding of these diseases. DAVFs represent a minority of intracranial vascular malformations. Although historically difficult to quantify, one study determined the detection rate of DAVF among adults to be 0.16/100,000 adults/year.[
The present series is the first to report multiple cases of DAVFs found incidentally during MMA embolization for the treatment of CSDH (10% of all comers in our series). As MMA embolization emerges as a more common treatment option, there is a growing need to recognize the presence of associated DAVF and adjust treatment strategies as needed.
CONCLUSION
We present three cases of incidentally discovered DAVFs during MMA embolization for the treatment CSDH. This series underscores the importance of evaluating for DAVF during initial angiography and making appropriate alterations in treatment strategy. Additional studies are warranted to further investigate the relationship between CSDH and DAVF, as the prevalence of high-grade DAVF might be higher than previously thought among select patients presenting with isolated subdural hematoma.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC. Prospective, population-based detection of intracranial vascular malformations in adults: The Scottish intracranial vascular malformation study (SIVMS). Stroke. 2003. 34: 1163-9
2. Bartek J, Sjåvik K, Ståhl F, Kristiansson H, Solheim O, Gulati S. Surgery for chronic subdural hematoma in nonagenarians: A Scandinavian population-based multicenter study. Acta Neurol Scand. 2017. 136: 516-20
3. Catapano JS, Nguyen CL, Wakim AA, Albuquerque FC, Ducruet AF. Middle meningeal artery embolization for chronic subdural hematoma. Front Neurol. 2020. 11: 557233
4. Cohen SD, Goins JL, Butler SG, Morris PP, Browne JD. Dural arteriovenous fistula: Diagnosis, treatment, and outcomes. Laryngoscope. 2009. 119: 293-7
5. Dumont TM, Rughani AI, Goeckes T, Tranmer BI. Chronic subdural hematoma: A sentinel health event. World Neurosurg. 2013. 80: 889-92
6. Freckmann N, Sartor K, Herrmann HD. Traumatic arteriovenous fistulae of the middle meningeal artery and neighbouring veins or dural sinuses. Acta Neurochir (Wien). 1981. 55: 273-81
7. Kim E. Refractory spontaneous chronic subdural hematoma: A rare presentation of an intracranial arteriovenous fistula. J Cerebrovasc Endovasc Neurosurg. 2016. 18: 373-8
8. Li G, Zhang Y, Zhao J, Zhu X, Yu J, Hou K. Isolated subdural hematoma secondary to Dural arteriovenous fistula: A case report and literature review. BMC Neurol. 2019. 19: 43
9. Maiuri F, Iaconetta G, Sardo L, Briganti F. Dural arteriovenous malformation associated with recurrent subdural haematoma and intracranial hypertension. Br J Neurosurg. 2001. 15: 273-6
10. Pappas CT, Zabramski JM, Shetter AG. Iatrogenic arteriovenous fistula presenting as a recurrent subdural hematoma. Case report. J Neurosurg. 1992. 76: 134-6
11. Reynolds MR, Lanzino G, Zipfel GJ. Intracranial dural arteriovenous fistulae. Stroke. 2017. 48: 1424-31
12. Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A. Middle meningeal artery embolization for chronic subdural hematoma: Meta-analysis and systematic review. World Neurosurg. 2019. 122: 613-9