- Division of Neurosurgery, Department of Surgery, Medical School, Federal University of Goiás, Goiânia, Brazil.
Correspondence Address:
Osvaldo Vilela-Filho, Division of Neurosurgery, Department of Surgery, Medical School, Federal University of Goiás, Goiânia, Brazil.
DOI:10.25259/SNI_246_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Osvaldo Vilela-Filho, Jairo Porfírio, Lissa C. Goulart. Indicators of correct targeting in stereotactic biopsy of intracranial lesions. 17-Jun-2022;13:251
How to cite this URL: Osvaldo Vilela-Filho, Jairo Porfírio, Lissa C. Goulart. Indicators of correct targeting in stereotactic biopsy of intracranial lesions. 17-Jun-2022;13:251. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11662
Abstract
Background: Confirmation of whether a stereotactic biopsy was performed in the correct site is usually dependent on the frozen section or on novel tumor-specific markers that are not widely available. Immediate postoperative computed tomography (CT) or magnetic resonance (MR) is routinely performed in our service after biopsy. In this retrospective study, we have carefully analyzed these images in an attempt to determine the presence of markers that indicate appropriate targeting.
Methods: Medical records and neuroimages of patients who underwent stereotactic biopsy of intracranial lesions were reviewed. The following variables were assessed: age, sex, anatomopathology, lesion site, complications, diagnostic accuracy, and the presence of image markers.
Results: Twenty-nine patients were included in this case series. About 96.6% of the biopsies were accurate according to the permanent section. Of the 86.2% of patients with intralesional pneumocephalus on the postoperative images, 51.7% additionally presented petechial hemorrhage. In 13.8% of the cases, no image markers were identified.
Conclusion: This is the first report of intralesional pneumocephalus and petechial hemorrhage as indicators of appropriate targeting in stereotactic biopsy. In the majority of the cases, an immediate postoperative head CT, which is widely available, can estimate how adequate the targeting is. To use intralesional pneumocephalus/ petechial hemorrhages as not only postoperative but also as intraoperative markers of appropriate targeting, it is advised that the surgical wound should be temporarily closed and dressed after the biopsy so that the patient can undergo a CT/MR scan and be checked for the presence of theses markers before removing the stereotactic frame.
Keywords: Brain tumor, Computed tomography, Magnetic resonance, Petechial hemorrhage, Pneumocephalus, Stereotactic biopsy
INTRODUCTION
Stereotactic brain biopsy has been used for many years to collect fragments of intracranial lesions that are usually deep seated or located in eloquent brain areas. It presents a high diagnostic accuracy, despite minimal invasion. The safety and reliability of this procedure are often paramount to the indication and optimization of therapies.[
The gold standard for intraoperative diagnosis of the acquired tissue samples is frozen section.[
Recently, new techniques that include pathologic tissue-specific markers, such as 5-aminolevulinic acid (5-ALA) and fluorescein, have been introduced, allowing intraoperative detection of fluorescence in the obtained sample, and thus providing prompt verification of targeting adequacy.[
Therefore, there seems to be a need to search for new markers that confirm whether the biopsied site was correct. Such markers should be, preferably, easily accessible and available at any neurosurgical center.
The main goal of this study was to verify the existence of these indicators on early postoperative computed tomography (CT) or magnetic resonance (MR) images of patients submitted to stereotactic biopsy of intracranial lesions.
MATERIALS AND METHODS
This is a retrospective study based on the review of medical records and neuroimaging studies of patients who underwent stereotactic biopsy of intracranial lesions performed by a single neurosurgeon (OV-F) at our institution, from May 2016 to October 2021. This study was approved by the Local Research Ethics Committee (technical report # 5.055.873) that waived the need for patient consent.
Early postoperative nonenhanced CT and/or MR images (first 24 h) were carefully analyzed for the presence of signs indicating appropriate lesion targeting and correlated with the preoperative images. The anatomopathology and immunohistochemistry reports were reviewed to determine the diagnostic yield. Procedure-related complications were recorded. Finally, the findings on the postoperative images were compared with the anatomopathology/ immunohistochemistry results.
Surgical technique
The stereotactic frame (AimSystem, Micromar, Diadema, Brazil) was placed with local anesthesia using a mixture of lidocaine 2% with vasoconstrictor and bupivacaine 0.5% (blockade of the greater and lesser occipital nerves and of the supraorbital nerves). A stereotactic post contrast CT scan was obtained and merged or not with frameless MR images (MNPS, Mevis, São Paulo, Brazil) acquired on the previous day, depending on the adequate identification of the lesion on CT images. The stereotactic coordinates, entry point, and trajectory of the biopsy needle were calculated using the aforementioned software. Sometimes, the coordinates were determined directly on the CT scanner using the available tools. The entry point varied according to the location of the lesion, but was placed at the precoronal parasagittal region with greater frequency.
The point elected for targeting depended on the following lesion characteristics: the margin and center of ring enhancing lesions; the center of homogeneous lesions; hotspots on MR perfusion; and areas with low apparent diffusion coefficient map on diffusion-weighted MR.
For tissue sampling, we used the Sedan needle with a lateral window of 5.0 or 10.0 mm, depending on the size of the lesion. The center of the lateral window was placed at the target. Specimens were usually collected at the four quadrants of the target, 5.0 mm above, and 5.0 mm below, totaling 12 samples.
The fragments were placed in a 10% formalin solution and sent to the pathology laboratory. Frozen section was not performed in these cases.
To rule out complications, a nonenhanced head CT or MR was performed up to 24 h after the procedure, more commonly within the first 6 h.
Inclusion criteria
Patients who underwent stereotactic biopsy at our institution, from May 2016 to October 2021, performed by a single neurosurgeon (OV-F), according to the technique described above.
Exclusion criteria
Unavailability of the pre- and/or postoperative CT or MR performed up to 24 h after the procedure, as well as of the pathology report.
Data availability
All the data reported in this manuscript are available on reasonable request from the corresponding author (OV-F). These data are not publicly available due to the risk of compromising patient privacy.
RESULTS
Thirty-eight patients fulfilled the inclusion criteria. Of those, nine were excluded based on missing postoperative neuroimaging studies for analysis. The remaining 29 patients were included in this study, being that 13 were male and 16 were female, with ages ranging from 20 to 79 (50.8 ± 17.9 years).
The planning of the stereotactic biopsy was based on CT alone in 23 patients and on CT/MR imaging fusion in six. Postoperatively, CT was performed in all patients and MR in six. Anatomopathology was performed in all patients and immunohistochemistry in 20.
A diagnosis could be established in 28 out of the 29 patients (96.6%), distributed as follows: low-grade gliomas, 20.7%; high-grade gliomas, 31.0%; metastases, 13.8%; primary lymphoma, 3.4%; autoimmune diseases, 17.2%; and infectious diseases, 10.3% [
The morbidity rate was 6.9%, presenting as a superficial wound infection in one patient and asymptomatic intracerebral hemorrhage (1.0 cm in diameter) at the biopsy site in another [
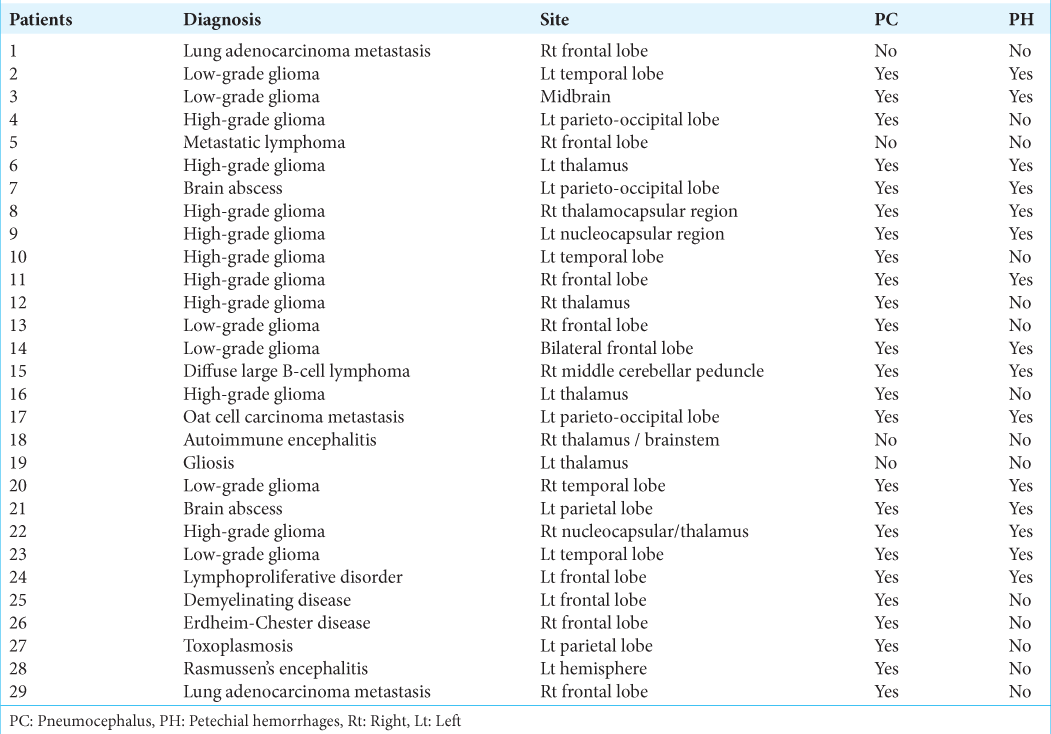
Figure 1:
(a) Preoperative stereotactic-enhanced CT showing an expansive lesion in the right nucleocapsular region and thalamus, presenting heterogeneous density, and contrast enhancement. The lesion was biopsied in three points (straight arrows): anterior part of the enhancing peripheral ring, anterior hypodense area, and enhanced solid region. (b and c) Postoperative unenhanced CT showing pneumocephalus (arrowheads), petechiae, and a small hemorrhage (curved arrows) at the biopsied sites. (d and e) Postoperative T2-weighted coronal (d) and sagittal (e) MR images showing the needle tracks (arrows) and the presence of hemosiderin (petechiae)/air (pneumocephalus) inside the lesion at the three biopsy sites. Diagnosis: high-grade glioma (patient #22).
There was no mortality in this case series.
Three patients underwent microsurgical resection of intracranial lesions, being the anatomopathology reports congruent with those of the stereotactic biopsy in all cases.
The evaluation of the postoperative neuroimaging studies revealed two distinct findings at the lesion (biopsy) site: pneumocephalus and petechial hemorrhage (defined as a hemorrhage with a diameter ≤5.0 mm, without mass effect). Pneumocephalus was observed in 25/29 patients (86.2%), being the only finding in 10 (10/29 = 34.5%) [
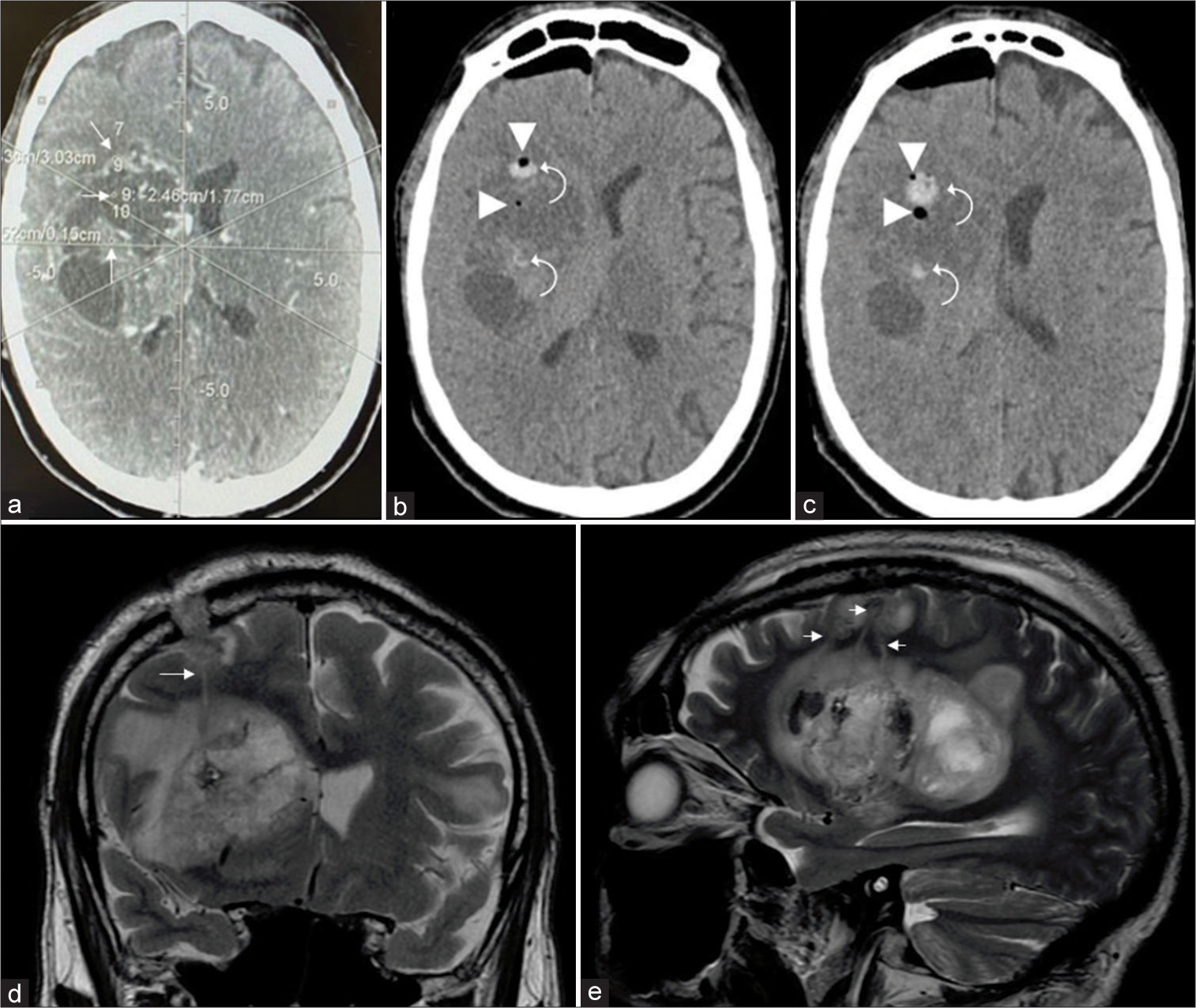
Figure 2:
(a) Preoperative unenhanced CT showing a hyperdense lesion in the right middle cerebellar peduncle and cerebellum. (b and c) Preoperative enhanced CT and MR showing that the lesion presents intense and homogeneous contrast enhancement. (d) Postoperative unenhanced CT demonstrating pneumocephalus (arrowhead) and petechial hemorrhage (curved arrow) at the biopsy site. Diagnosis: diffuse large B-cell lymphoma (patient #15).
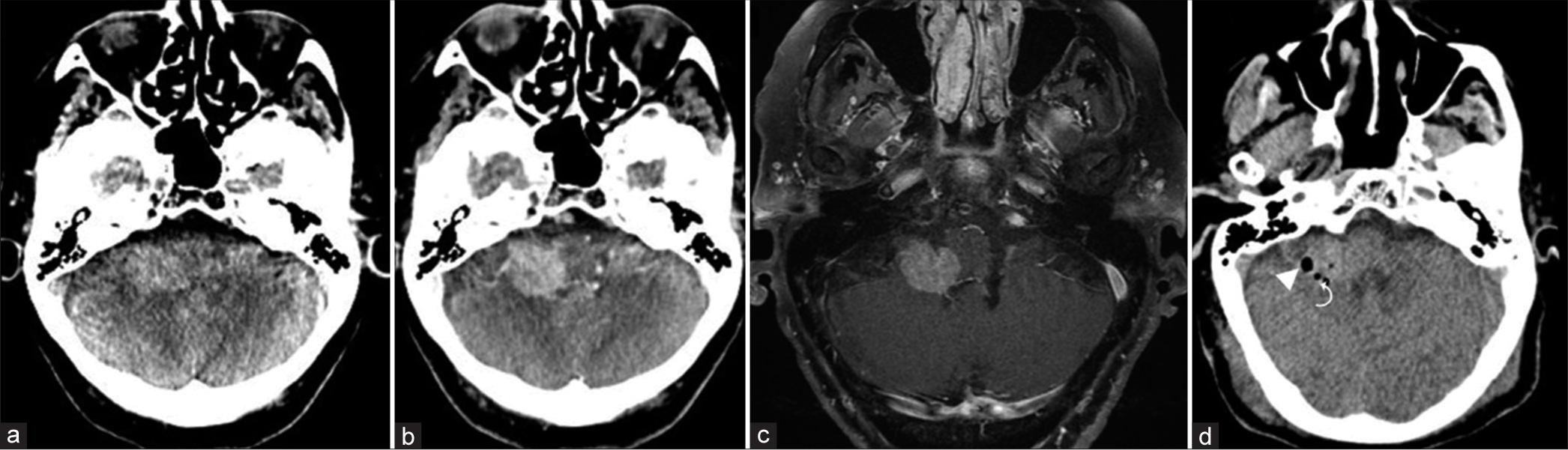
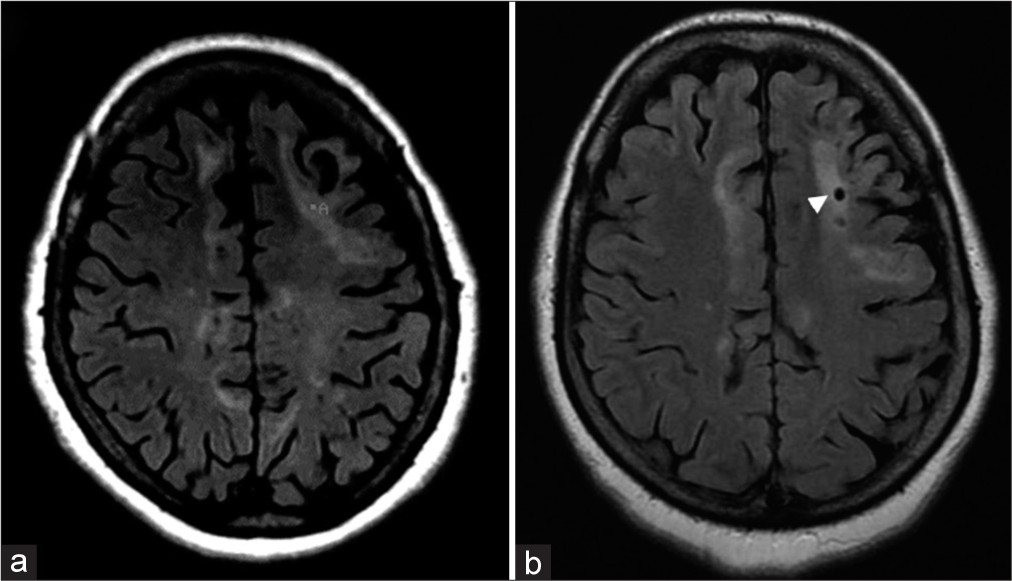
Figure 4:
(a) Preoperative FLAIR MR image showing various areas of high signal in the subcortical/juxtacortical white matter of the left frontal lobe. “A” indicates the target chosen for stereotactic biopsy. (b) Postoperative FLAIR MR showing pneumocephalus at the biopsy site (arrowhead). Diagnosis: demyelinating disease (patient #25).
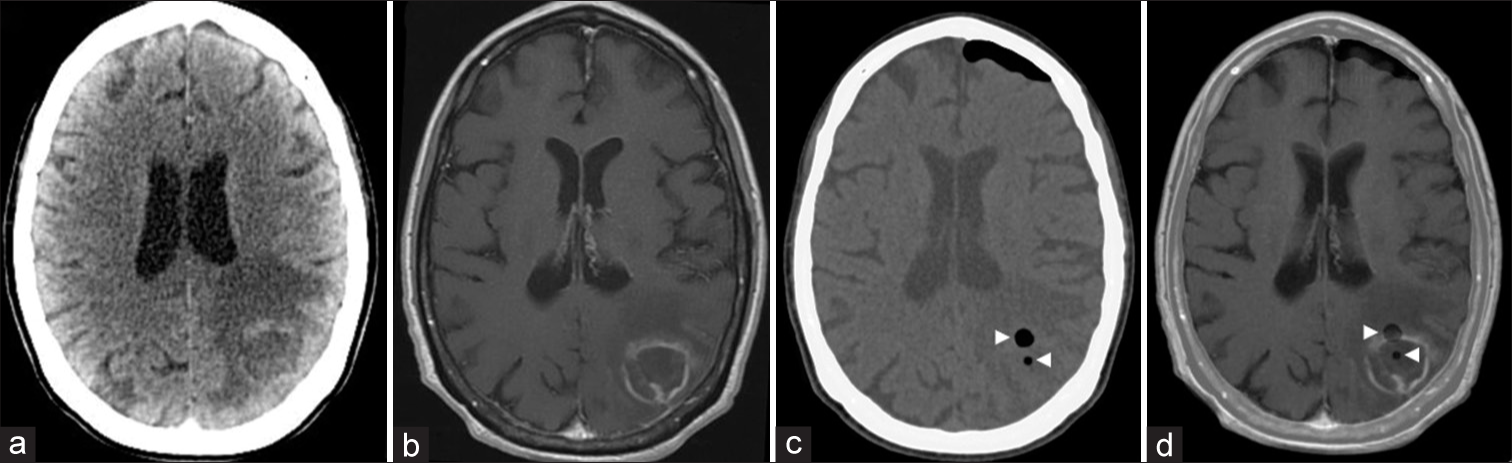
Figure 5:
(a and b) Preoperative enhanced CT and T1-weighted MR axial images showing a ring enhancing lesion in the left parietal lobe, with a hypodense/hypointense central region, and marked vasogenic edema in the adjacent parenchyma. Both the central and anterior parts of the enhanced ring were targeted. (c) Postoperative unenhanced CT with two foci of pneumocephalus (arrowheads) at the biopsy sites. (d) Postoperative unenhanced CT merged with the preoperative enhanced T1-weighted MR image showing two foci of pneumocephalus (arrowheads) at the two biopsy sites: central and peripheral enhancing ring regions of the lesion. Diagnosis: toxoplasmosis (patient #27).
DISCUSSION
Stereotactic biopsy allows the neurosurgeon to diagnose lesions in any location, by the safe acquirement of small samples. It is a tool of utmost importance for the diagnosis of intracranial lesions, particularly those with a difficult approach or located in eloquent areas, ensuring great accuracy and, therefore, adequate treatment.[
The accuracy rate in the present study was 96.6%, which is within the range reported by other authors (87.6–100%).[
The gold standard to determine the accuracy of the stereotactic biopsy is frozen section, as it allows prompt diagnosis and verification if the biopsy was performed at the intended site.[
In cases of brain abscess or cystic lesions, aspiration of pus or fluid is sufficient to confirm targeting.
Newer techniques, such as fluorescein[
Intraoperative MR or CT may also be used to confirm appropriate targeting.[
Therefore, in institutions where frozen section is not part of the routine in stereotactic biopsy and intraoperative MR/CT or the fluorescein/5-ALA techniques are unavailable, there is a need to determine some widely accessible indicators of proper targeting. In our series, pneumocephalus, accompanied or not by petechial hemorrhage, at the biopsy site, presents on neuroimaging studies performed within 24 h of surgery, proved to be reliable indicators, being found in 86.2% of the cases (25/29). The permanent section was conclusive in all of the cases with pneumocephalus (25/25), but only in 75% of the procedures where these indicators were not present (3/4). It is possible that the high frequency of these findings is related with the number of specimens (n = 12) collected during the procedure, but as previously mentioned, it did not increase the morbidity rate. Therefore, it is our belief that the petechial hemorrhages, along with the pneumocephalus at the lesion (biopsy) site, always asymptomatic, should be regarded as indicators of proper targeting, and not as procedure-related complications.
In cases of lesions identified only on MR images, a postoperative MR, instead of CT, is mandatory to confirm the presence of these markers. Alternatively, one could merge postoperative CT with preoperative MR images to achieve the same goal.
The presence of these findings exclusively outside, but near the lesion site, is probably indicative of inadequate targeting, which should raise a red flag and indicate the need for another biopsy even before the result of the permanent section. The same may not be said when these indicators are not present, though, since it happened in four of our patients and the permanent section was conclusive in three of them.
One may argue that it is necessary to know whether the targeting is adequate during surgery and not afterward, once the stereotactic frame has already been removed. Considering that, if one has no accessibility to frozen section, 5-ALA/ fluorescein or intraoperative CT/MR, we suggest, after tissue sampling and before the frame is removed, to temporarily close and dress the surgical wound, take the patient to the CT/MR suite, and check for the presence of intralesional pneumocephalus/petechial hemorrhage.
It should be highlighted that this study does not allow us to conclude for how long the markers may be observed since all postoperative imaging studies were performed within 24 h from surgery. In addition, we cannot say if they are present when fewer specimens are collected for permanent section. In this regard, it is relevant to mention that, in a single patient, biopsy was performed at two distinct sites and only four samples were collected from one of them. Nevertheless, intralesional pneumocephalus occurred at the two sites and a head CT, repeated 5 days after the procedure, due to a complaint of headache, demonstrated that the pneumocephalus was still present.
CONCLUSION
This study describes, for the 1st time, two markers, intralesional pneumocephalus and petechial hemorrhage that are easily identifiable on immediate postoperative CT and/or MR, indicating appropriate targeting in stereotactic biopsy of brain lesions. These markers were present in 86.2% of our patients and permanent section was diagnostic in all of them. The use of this technique has some significant advantages: it cuts down the surgical time, is widely available, is highly accurate, and apparently dispenses the use of other more expensive and not universally available techniques such as frozen section, 5-ALA, fluorescein, and intraoperative CT or MR. To use intralesional pneumocephalus/petechial hemorrhages not only as postoperative but also rather as intraoperative markers of appropriate targeting, it would be advisable, after the biopsy and before the frame is removed, to temporarily close and dress the surgical wound, take the patient to the CT/MR suite, and check for the presence of these markers.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to express their gratitude to Bernardo Drummond, MD, PhD, and Luiz A. Ferreira-Filho, MD, for their support.
References
1. Abdelaziz O, Eshra M, Belal A, Elshafei M. Diagnostic value of magnetic resonance spectroscopy compared with stereotactic biopsy of intra-axial brain lesions. J Neurol Surg A Cent Eur Neurosurg. 2016. 77: 283-90
2. Æbelø AM, Noer VR, Schulz MK, Kristensen BW, Pedersen CB, Poulsen FR. Frameless stereotactic neuronavigated biopsy: A retrospective study of morbidity, diagnostic yield, and the potential of fluorescence. Clin Neurol Neurosurg. 2019. 181: 28-32
3. Akay A, Işlekel S. MRI-guided frame-based stereotactic brainstem biopsy procedure: A single-center experience. Neurocirugía. 2019. 30: 167-72
4. Akay A, Rüksen M, Islekel S. Magnetic resonance imaging-guided stereotactic biopsy: A review of 83 cases with outcomes. Asian J Neurosurg. 2019. 14: 90-5
5. Amraei R, Moradi A, Zham H, Ahadi M, Baikpour M, Rakhshan A. A Comparison between the diagnostic accuracy of frozen section and permanent section analyses in central nervous system. Asian Pac J Cancer Prev. 2017. 18: 659-66
6. Bahrami E, Parvaresh M, Bahrami M, Fattahi A. An experience with frame-based stereotactic biopsy of posterior fossa lesions via transcerebellar route. World Neurosurg. 2020. 136: e380-5
7. Bander ED, Jones SH, Pisapia D, Magge R, Fine H, Schwartz TH. Tubular brain tumor biopsy to improve diagnostic yield for subcortical lesions. J Neurooncol. 2019. 141: 121-9
8. Barkley AS, Sullivan LT, Gibson AW, Camacho D, Barber JK, Ko AL. Stereotactic brain biopsy hemorrhage risk factors and implications for postoperative care at a single institution: An argument for postoperative imaging. World Neurosurg. 2020. 144: 807-12
9. Bernays RL, Kollias SS, Khan N, Brandner S, Meier S, Yonekawa Y. Histological yield, complications, and technological considerations in 114 consecutive frameless stereotactic biopsy procedures aided by open intraoperative magnetic resonance imaging. J Neurosurg. 2002. 97: 354-62
10. Beynon C, Wei S, Radbruch A, Capper D, Unterberg AW, Kiening KL. Preoperative assessment of haemostasis in patients undergoing stereotactic brain biopsy. J Clin Neurosci. 2018. 53: 112-6
11. Bishokarma S, Shrestha S, Napit M, Gongal DN. Clinical experience with frame based stereotactic biopsy for intracranial space occupying lesion. JNMA J Nepal Med Assoc. 2018. 56: 749-53
12. Callovini GM, Telera S, Sherkat S, Sperduti I, Callovini T, Carapella CM. How is stereotactic brain biopsy evolving? A multicentric analysis of a series of 421 cases treated in Rome over the last sixteen years. Clin Neurol Neurosurg. 2018. 174: 101-7
13. Can S, Turkmenoglu O, Tanik C, Uysal E, Ozoner B, Kaldirimoglu S. Computerized tomography-guided stereotactic biopsy of intracranial lesions: Report of 500 consecutive cases. Turk Neurosurg. 2017. 27: 395-400
14. Catapano G, Sgulò FG, Seneca V, Iorio G, de Notaris M, di Nuzzo G. Fluorescein-assisted stereotactic needle biopsy of brain tumors: A single-center experience and systematic review. Neurosurg Rev. 2019. 42: 309-18
15. Chen CC, Hsu PW, Wu TW, Lee ST, Chang CN, Wei KC. Stereotactic brain biopsy: Single center retrospective analysis of complications. Clin Neurol Neurosurg. 2009. 111: 835-9
16. Cheng G, Yu X, Zhao H, Cao W, Li H, Li Q. Complications of stereotactic biopsy of lesions in the sellar region, pineal gland, and brainstem: A retrospective, single-center study. Medicine (Baltimore). 2020. 99: e18572
17. Citterio G, Reni M, Gatta G, Ferreri AJ. Primary central nervous system lymphoma. Crit Rev Oncol Hematol. 2017. 113: 97-110
18. Cordone I, Masi S, Carosi M, Vidiri A, Marchesi F, Marino M. Brain stereotactic biopsy flow cytometry for central nervous system lymphoma characterization: Advantages and pitfalls. J Exp Clin Cancer Res. 2016. 35: 128
19. Di N, Cheng W, Jiang X, Liu X, Zhou J, Xie Q. Can dynamic contrast-enhanced MRI evaluate VEGF expression in brain glioma? An MRI-guided stereotactic biopsy study. J Neuroradiol. 2019. 46: 186-92
20. Furtak J, Mielczarek M, Szylberg M, Harat M. Biomarker concordance between molecular stereotactic biopsy and open surgical specimens in gliomas. Neurol Neurochir Pol. 2019. 53: 435-41
21. Georgiopoulos M, Ellul J, Chroni E, Constantoyannis C. Efficacy, safety, and duration of a frameless fiducial-less brain biopsy versus frame-based stereotactic biopsy: A prospective randomized study. J Neurolog Surg A Cent Eur Neurosurg. 2018. 79: 31-8
22. Hamisch C, Blau T, Klinger K, Kickingereder P, Ruess D, Galldiks N. Feasibility, risk profile and diagnostic yield of stereotactic biopsy in children and young adults with brain lesions. Klin Padiatr. 2017. 229: 133-41
23. Hamisch C, Kickingereder P, Fischer M, Simon T, Ruge M. Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: A systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr. 2017. 20: 261-8
24. Hamisch C, Minartz J, Blau T, Hafkemeyer V, Rueß D, Hellerbach A. Frame-based stereotactic biopsy of deep-seated and midline structures in 511 procedures: Feasibility, risk profile, and diagnostic yield. Acta Neurochir. 2019. 16: 2065-71
25. Janjua MB, Ban VS, El Ahmadieh TY, Hwang SW, Samdani AF, Price AV. Diffuse intrinsic pontine gliomas: Diagnostic approach and treatment strategies. J Clin Neurosci. 2020. 72: 15-9
26. Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. F1000Res. 2018. 7: F1000
27. Lara-Almunia M, Hernandez-Vicente J. Frame-based stereotactic biopsy: Description and association of anatomical, radiologic, and surgical variables with diagnostic yield in a series of 407 cases. J Neurol Surg A Cent Eur Neurosurg. 2019. 80: 149-61
28. Lara-Almunia M, Hernandez-Vicente J. Symptomatic intracranial hemorrhages and frame-based stereotactic brain biopsy. Surg Neurol Int. 2020. 11: 218
29. Lu CY, Xu ZS, Ye X. Evaluation of intraoperative MRI-assisted stereotactic brain tissue biopsy: A single-center experience in China. Chin Neurosurg J. 2019. 5: 4
30. Lynagh R, Ishak M, Georges J, Lopez D, Osman H, Kakareka M. Fluorescence-guided stereotactic biopsy: A proof-of-concept study. J Neurosurg. 2019. 132: 1-7
31. Manjila S, Knudson KE, Johnson C, Sloan AE. Monteris AXiiiS stereotactic miniframe for intracranial biopsy: Precision, feasibility, and ease of use. Oper Neurosurg. 2016. 12: 119-27
32. Maragkos GA, Penumaka A, Ahrendsen JT, Salem MM, Nelton EB, Alterman RL. Factors affecting the diagnostic yield of frame-based stereotactic intracranial biopsies. World Neurosurg. 2020. 135: 695-701
33. Markwardt NA, Stepp H, Franz G, Sroka R, Goetz M, Zelenkov P. Remission spectrometry for blood vessel detection during stereotactic biopsy of brain tumors. J Biophotonics. 2017. 10: 1080-94
34. Mathon B, Amelot A, Mokhtari K, Bielle F. Increasing the diagnostic yield of stereotactic brain biopsy using intraoperative histological smear. Clin Neurol Neurosurg. 2019. 186: 105544
35. Millesi M, Kiesel B, Wöhrer A, Mercea PA, Bissolo M, Roetzer T. Is intraoperative pathology needed if 5-aminolevulinic-acid-induced tissue fluorescence is found in stereotactic brain tumor biopsy?. Neurosurgery. 2020. 86: 366-73
36. Millesi M, Widhalm G. Is intraoperative pathology needed if 5-aminolevulinic-acid-induced tissue fluorescence is found in stereotactic brain tumor biopsy?. Neurosurgery. 2020. 87: E427
37. Mizobuchi Y, Nakajima K, Fujihara T, Matsuzaki K, Mure H, Nagahiro S. The risk of hemorrhage in stereotactic biopsy for brain tumors. J Med Invest. 2019. 66: 314-8
38. Mohyeldin A, Lonser RR, Elder JB. Real-time magnetic resonance imaging-guided frameless stereotactic brain biopsy: Technical note. J Neurosurg. 2016. 124: 1039-46
39. Morell AA, Shah AH, Cavallo C, Eichberg DG, Sarkiss CA, Benveniste R. Diagnosis of primary central nervous system lymphoma: A systematic review of the utility of CSF screening and the role of early brain biopsy. Neurooncol Pract. 2019. 6: 415-23
40. Moreno-Jiménez S, Martínez-Vaca N, Pérez-Aguilar B, GómezCalva B, Díaz-Chávez J, Soto-Mondragón M. Usefulness and safety from stereotactic biopsy in posterior fossa lesions in adult patients. Cir Cir. 2019. 87: 554-8
41. Nikoobakht M, Shamshiripour P, Nekoo ZA, Haghmohammadi SF. Elevated Lactate and total protein levels in stereotactic brain biopsy specimen; potential biomarkers of malignancy and poor prognosis. Arch Iran Med. 2019. 22: 125-31
42. Pennlund A, Jakola AS, Skoglund T, Ljungqvist J. A single-centre study of frame-based stereotactic brain biopsies. Br J Neurosurg. 2021. 10: 1-4
43. Qin F, Huang ZC, Cai MQ, Xu X, Lu TT, Dong Q. Stereotactic biopsy in the accurate diagnosis of lesions in the brain Stem and deep brain. Zhonghua Yi Xue Za Zhi. 2018. 98: 1771-4
44. Quick-Weller J, Kann G, Lescher S, Imöhl L, Seifert V, Weise LM. Impact of stereotactic biopsy in HIV patients. World Neurosurg. 2016. 86: 300-5
45. Quick-Weller J, Lescher S, Baumgarten P, Dinc N, Bruder M, Weise L. Stereotactic biopsy of pineal lesions. World Neurosurg. 2016. 96: 124-8
46. Quick-Weller J, Lescher S, Bruder M, Dinc N, Behmanesh B, Seifert V. Stereotactic biopsy of brainstem lesions: 21 years experiences of a single center. J Neurooncol. 2016. 129: 243-50
47. Quick-Weller J, Tichy J, Dinc N, Tritt S, Won S, Behmanesh B. Benefit and complications of frame-based stereotactic biopsy in old and very old patients. World Neurosurg. 2017. 102: 442-8
48. Quick-Weller J, Tichy J, Harter PN, Tritt S, Baumgarten P, Bähr O. Two is not enough” Impact of the number of tissue samples obtained from stereotactic brain biopsies in suspected glioblastoma. J Clin Neurosci. 2018. 47: 311-4
49. Riche M, Amelot A, Matthieu P, Capelle L, Carpentier A, Mathon B. Complications after frame-based stereotactic brain biopsy: A systematic review. Neurosurg Rev. 2019. 44: 301-7
50. Saß B, Pojskic M, Bopp M, Nimsky C, Carl B. Comparing fiducial-based and intraoperative computed tomography-based registration for frameless stereotactic brain biopsy. Stereotact Funct Neurosurg. 2021. 99: 79-89
51. Sciortino T, Fernandes B, Nibali MC, Gay LG, Rossi M, Lopci E. Frameless stereotactic biopsy for precision neurosurgery: Diagnostic value, safety, and accuracy. Acta Neurochir. 2019. 161: 967-74
52. Shofty B, Richetta C, Haim O, Kashanian A, Gurevich A, Grossman R. 5-ALA-assisted stereotactic brain tumor biopsy improve diagnostic yield. Eur J Surg Oncol. 2019. 45: 2375-8
53. Taweesomboonyat C, Tunthanathip T, Sae-Heng S, Oearsakul T. Diagnostic yield and complication of frameless stereotactic brain biopsy. J Neurosci Rural Pract. 2019. 10: 78-84
54. Thien A, Han JX, Kumar K, Ng YP, Rao JP, Ng WH. Investigation of the usefulness of fluorescein sodium fluorescence in stereotactic brain biopsy. Acta Neurochir. 2018. 160: 317-24
55. Thien A, Rao JP, Ng WH, King NK. The Fluoropen: A simple low-cost device to detect intraoperative fluorescein fluorescence in stereotactic needle biopsy of brain tumors. Acta Neurochir. 2017. 159: 371-5
56. Todeschi J, Bund C, Cebula H, Chibbaro S, Lhermitte B, Pin Y. Diagnostic value of fusion of metabolic and structural images for stereotactic biopsy of brain tumors without enhancement after contrast medium injection. Neurochirurgie. 2019. 65: 357-64
57. Yu KKH, Patel AR, Moss NS. The role of stereotactic biopsy in brain metastases. Neurosurg Clin N Am. 2020. 31: 515-26
58. Zhang J, Liu X, Fu K, Xu C, Gong R, Liu L. Diagnostic value and safety of stereotactic biopsy in acquired immune deficiency syndrome patients with intracranial lesions: Systematic review and meta-analysis. World Neurosurg. 2017. 98: 790-9.e13
59. Zhao WJ, Chen JZ, Wang GZ, Yu Q, He P, Jia Y. Stereotactic brain biopsy guided by iMRI co-registration combined with PET/CT. Zhonghua Yi Xue Za Zhi. 2016. 96: 685-8