- Department of Neurosurgery, Roswell Park Comprehensive Cancer Center, Buffalo, New York, United States,
- Department of Neurosurgery, Jacobs School of Medicine at the University at Buffalo, Buffalo New York, United States.
Correspondence Address:
Lindsay J. Lipinski Department of Neurosurgery, Roswell Park Comprehensive Cancer Center, Jacobs School of Medicine at the University at Buffalo, Buffalo, New York, United States.
DOI:10.25259/SNI_345_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Matthew J. Recker1,2, Steven B. Housley1,2, Lindsay J Lipinski1,2. Indolent nonendemic central nervous system histoplasmosis presenting as an isolated intramedullary enhancing spinal cord lesion. 09-Aug-2021;12:392
How to cite this URL: Matthew J. Recker1,2, Steven B. Housley1,2, Lindsay J Lipinski1,2. Indolent nonendemic central nervous system histoplasmosis presenting as an isolated intramedullary enhancing spinal cord lesion. 09-Aug-2021;12:392. Available from: https://surgicalneurologyint.com/surgicalint-articles/11036/
Abstract
Background: Histoplasma capsulatum infection is largely seen in endemic regions; it results in symptomatic disease in
Case Description: We present an atypical case of a 44-year-old man from a nonendemic region, on adalimumab therapy for ulcerative colitis who developed an isolated intramedullary spinal cord lesion in the setting of disseminated histoplasmosis. His course was initially indolent with vague systemic symptoms that led to consideration of several other diagnoses including sarcoidosis and lymphoma. Biopsies of several positron emission tomography positive lymph nodes revealed granulomatous inflammation, but no firm diagnosis was achieved. He was ultimately diagnosed with histoplasmosis after an acute respiratory infection in the setting of anti-tumor necrosis factor therapy. With appropriate antifungal therapy, the spinal cord lesion regressed. The previous systemic biopsies were re-reviewed, and rare fungal elements consistent with H. capsulatum were identified. A presumptive diagnosis of CNS histoplasmosis was made in the absence of direct laboratory confirmation in the setting of rapid and complete resolution on antifungal therapy.
Conclusion: Disseminated histoplasmosis should be considered in granulomatous disease, even if the patient resides in a nonendemic region. Furthermore, clinicians should be mindful that CNS histoplasmosis may present in an atypical fashion.
Keywords: Abscess, Central nervous system, Histoplasmosis, Intramedullary, Spinal cord
INTRODUCTION
Although infection with the fungus Histoplasma capsulatum is common – especially in the Ohio and Mississippi River Valleys, where the pathogen is endemic in the United States – histoplasmosis disease results in <5% of those infected.[
CASE DESCRIPTION
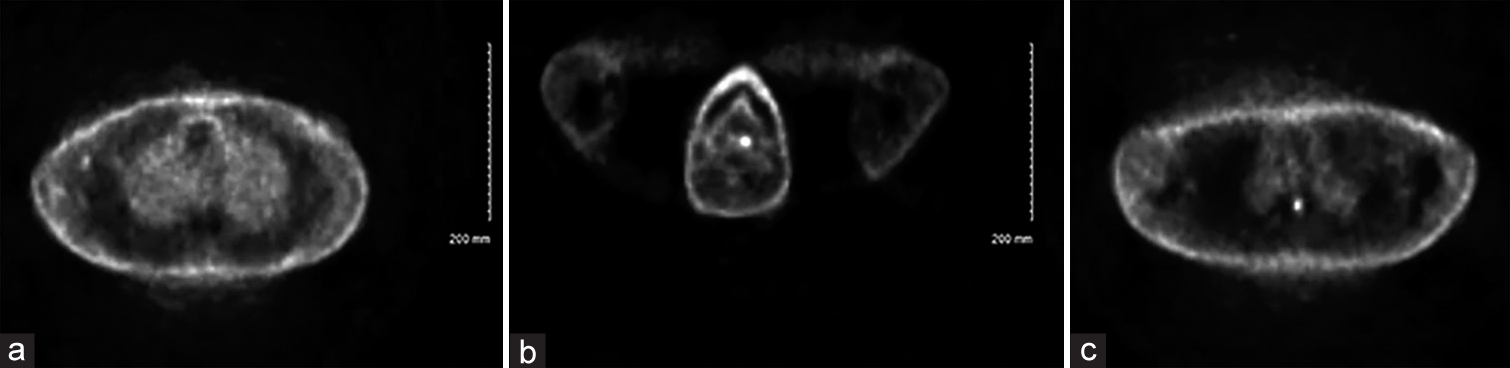
A previously healthy 44-year-old man from the Great Lakes region of the United States with a medical history remarkable for ulcerative colitis on adalimumab therapy presented a 3–4 month history of abdominal pain, weight loss, night sweats, and fatigue. Workup for evaluation of these symptoms included colonoscopy, which demonstrated areas of inflammation and ulceration. Colonic biopsies were interpreted as consistent with acute colitis. Approximately 1 month later, computed tomography of the abdomen and pelvis, which was performed due to ongoing and somewhat progressive symptoms, demonstrated abnormal mesenteric lymph nodes. Follow-up positron emission tomography (PET) imaging revealed low-grade metabolic activity in mesenteric, aortocaval, internal mammary, abdominal wall, and axillary nodes as well as heterogeneous uptake in the spleen consistent with “low-grade lymphoproliferative disease.” Additional hypermetabolic lesions were observed in the left lingual tonsil and within the spinal cord at the level of the third thoracic vertebra [
The patient subsequently underwent an ultrasound-guided biopsy of a PET-avid periumbilical abdominal wall mass, a PET-avid cervical node, and a PET-avid lingual tonsil, which all showed no evidence of malignancy on pathology but each contained granulomatous inflammation. Both necrotizing and non-necrotizing granulomas were identified in the specimens. Around this time, the patient developed clumsiness and numbness of his left-lower extremity. Magnetic resonance imaging (MRI) of the spine demonstrated a 9-mm expansile homogeneously enhancing intramedullary spinal cord mass at the T3 level with significant edema within the spinal cord above and below the lesion [
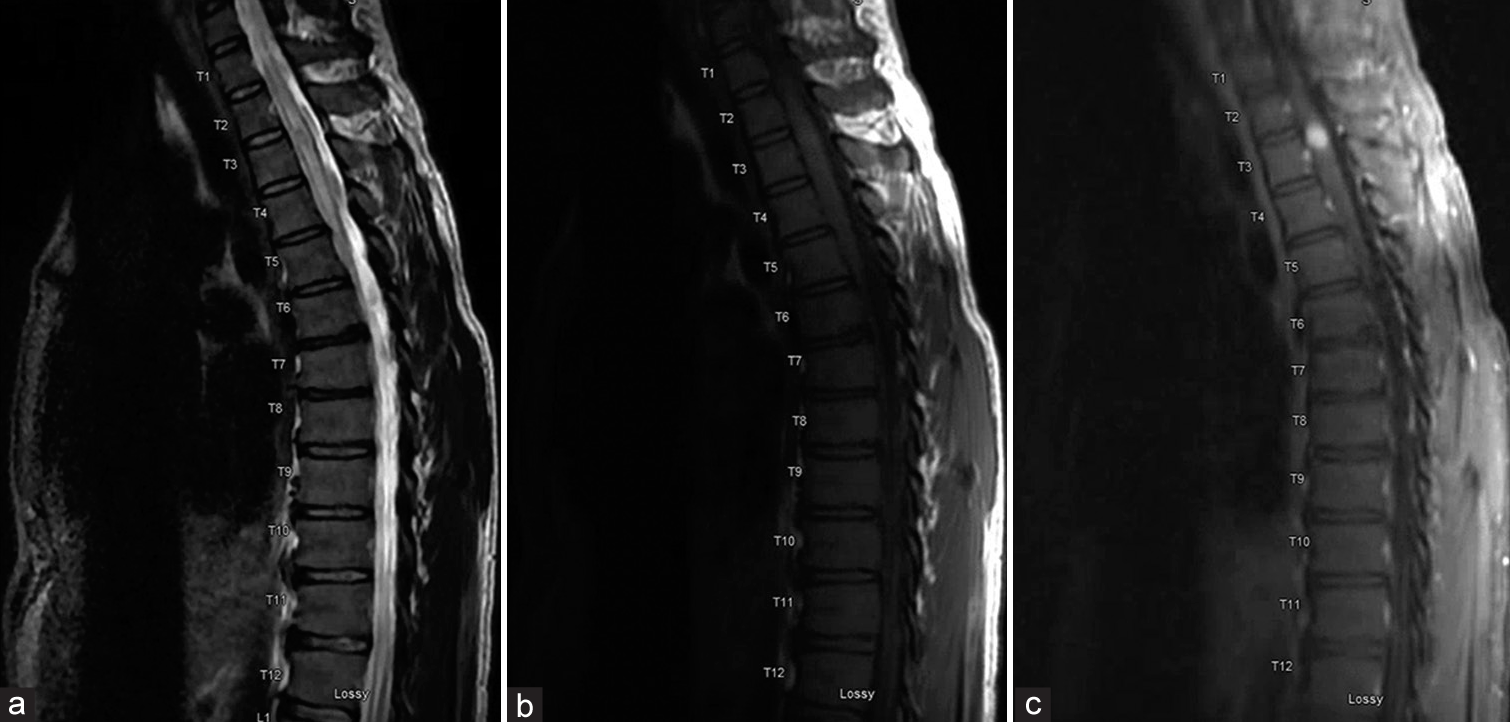
Figure 2:
Initial magnetic resonance imaging of the thoracic spine demonstrates a 9-mm homogeneously-enhancing intramedullary spinal cord lesion with significant edema within the spinal cord above and below the lesion. Sagittal T2-weighted image (a), sagittal T1-weighted image without contrast (b), and sagittal T1-weighted image without contrast (c) are shown.
In the absence of a systemic diagnosis, the patient was referred to pulmonology for possible multisystem sarcoidosis, where he was treated with high-dose prednisone therapy. The patient continued this therapy for approximately 3 months during which time he reported improvements in his appetite, strength, night sweats, and chills. A repeat MRI was performed to follow the thoracic lesion, which showed significant improvement [
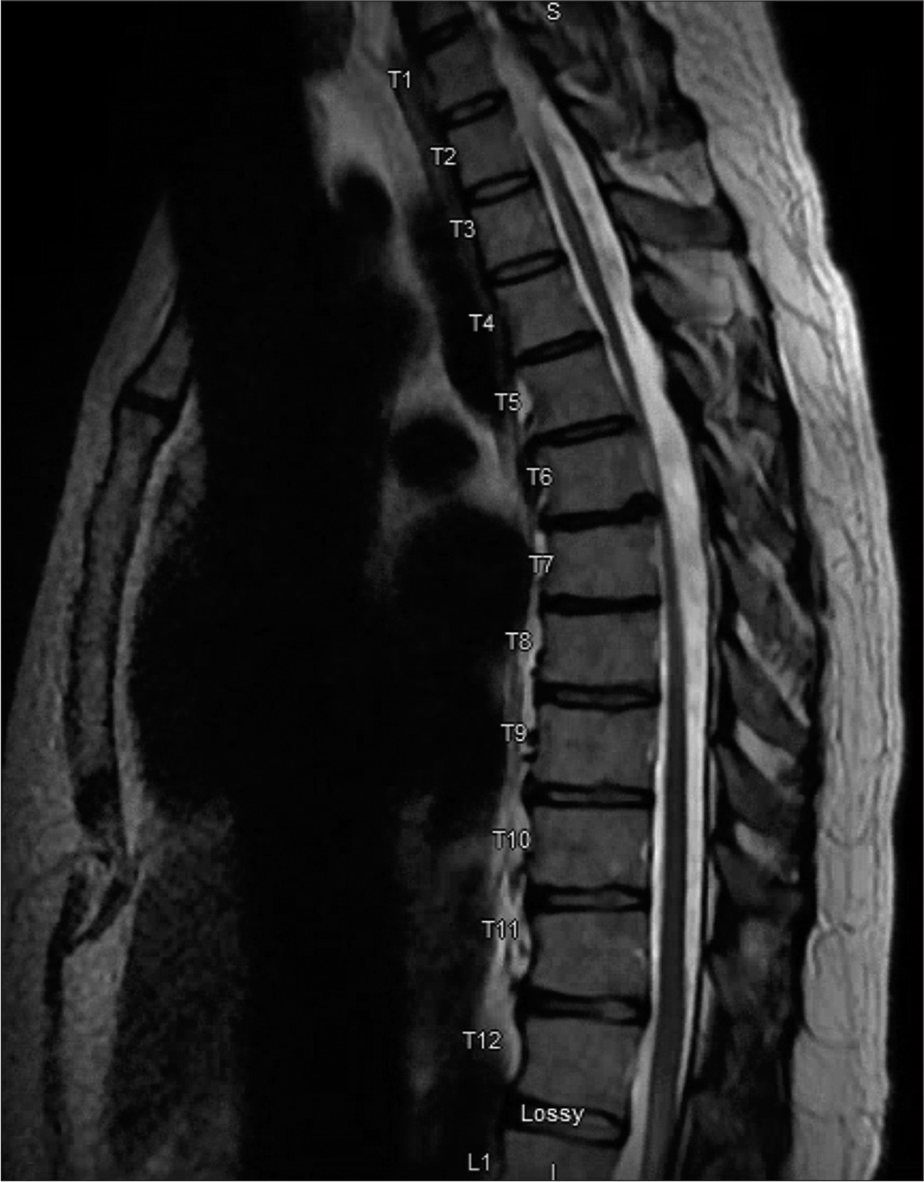
Figure 3:
Follow up serial sagittal T1-weighted magnetic resonance imaging of the thoracic spine with contrast – 3 months (a), 6 months (b), and 10 months (c) from initial imaging [Figure 2c] – demonstrate interval regression and ultimate resolution of the enhancing intramedullary spinal cord lesion.
Over the ensuing 3 months, his prednisone dose was slowly tapered down, and a course of intravenous infliximab was initiated for treatment of both presumed sarcoidosis and ulcerative colitis. Follow-up MRI of the thoracic lesion again showed significant reduction in size and patient reported continued improvement in strength and sensation of his left leg [
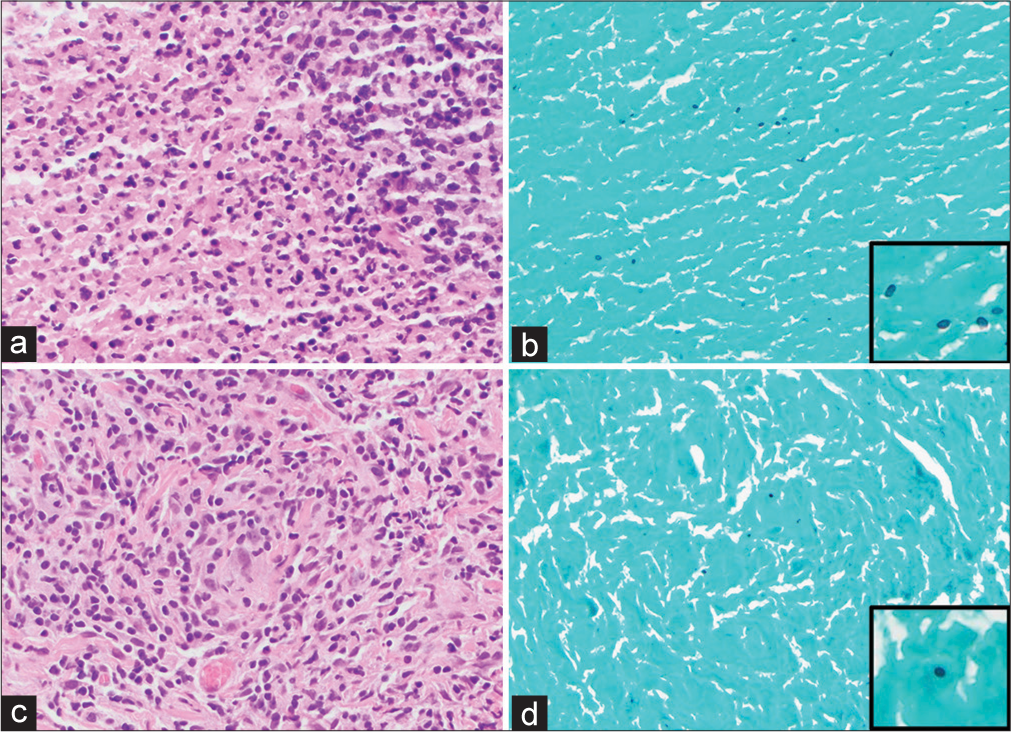
Figure 5:
Hematoxylin and eosin (H&E) (a) and Grocott methenamine silver (GMS) special stain (b) (×400) of the necrotizing granuloma in the left tonsil. H&E (c) and GMS special stain (d) (×400) of the non-necrotizing granuloma in the abdominal wall. A few GMS-positive fungal microorganisms are identified that are morphologically consistent with histoplasma.
DISCUSSION
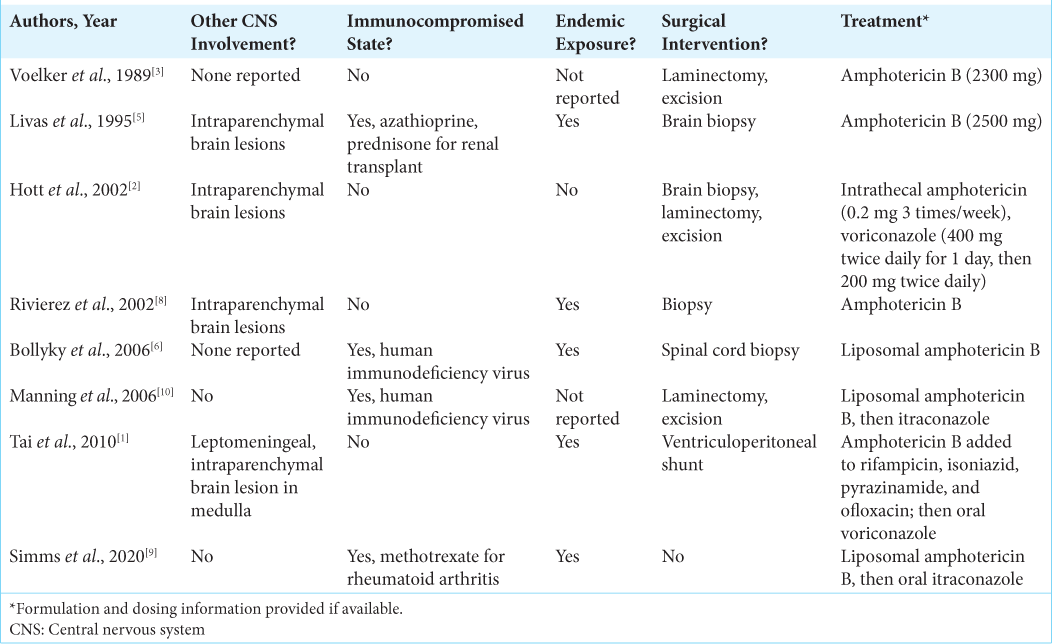
Histoplasmosis is most commonly a self-limited respiratory disease primarily seen in endemic regions. Disseminated disease is primarily seen in immunocompromised hosts and can involve the CNS. CNS involvement limited to the spinal cord as an isolated lesion is rarely described. Spinal cord involvement of histoplasmosis has been described in case reports and series. The
The diagnosis of CNS histoplasmosis can be challenging; and delays in diagnosis as well as misdiagnoses such as lymphoma, sarcoidosis, and other systemic inflammatory diseases have been described.[
Our patient’s CNS manifestation was unique in that CNS histoplasmosis uncommonly presents as an isolated spinal cord lesion.[
The indolent disease course in our patient should be noted. He was retrospectively found to have disseminated histoplasmosis at the time of the initial tissue biopsy from his colonoscopy, implying a prolonged infection that was slowly progressive even without antifungal therapy and while on immunosuppressive therapy. He was subsequently treated with a course of high-dose prednisone for presumed sarcoidosis, during which he radiographically and symptomatically improved from a neurologic standpoint. He was then prescribed a different anti-TNF agent which likely contributed to his ultimate development of acute pulmonary histoplasmosis. However, his pulmonary presentation occurred months into the therapy. Once the diagnosis of disseminated acute on chronic histoplasmosis was established, appropriate antifungal treatment was initiated, which led to complete radiographic resolution of the spinal cord lesion.
CONCLUSION
CNS histoplasmosis can be difficult to diagnose because atypical features can be common; it can closely mimic other systemic diseases, and the more sensitive diagnostic modalities are likely underutilized. CNS histoplasmosis should be considered even in nonendemic areas, with or without a history of immunosuppression, and even in the setting of an isolated enhancing spinal cord lesion. Clinicians must be mindful that histoplasmosis may appear radiographically similar to neurosarcoidosis and other systemic diseases and that treatment with immunosuppressive agents may unmask a previously indolent infection. Treatment with steroids may lead to clinical and radiographic improvement in CNS histoplasmosis. If granulomas are identified without clear etiology, fungal infection should be considered and a thorough search for fungal elements may be necessary.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bollyky PL, Czartoski TJ, Limaye A. Histoplasmosis presenting as an isolated spinal cord lesion. Arch Neurol. 2006. 63: 1802-3
2. Hott JS, Horn E, Sonntag VK, Coons SW, Shetter A. Intramedullary histoplasmosis spinal cord abscess in a nonendemic region: Case report and review of the literature. J Spinal Disord Tech. 2003. 16: 212-5
3. Livas IC, Nechay PS, Nauseef WM. Clinical evidence of spinal and cerebral histoplasmosis twenty years after renal transplantation. Clin Infect Dis. 1995. 20: 692-5
4. Manning TC, Born D, Tredway TL. Spinal intramedullary histoplasmosis as the initial presentation of human immunodeficiency virus infection: Case report. Neurosurgery. 2006. 59: E1146
5. Mehta AC, Ali SR. Mnemonic for the differential diagnosis of non-caseating granulomas. Sarcoidosis Vasc Diffuse Lung Dis. 2017. 34: 200-7
6. Rivierez M, Heyman D, Brebion A, Landau-Ossondo M, Desbois N, Vally P. Spinal cord histoplasmoma. A case report. Neurochirurgie. 2002. 48: 44-8
7. Saccente M. Central nervous system histoplasmosis. Curr Treat Options Neurol. 2008. 10: 161-7
8. Simms A, Kobayashi T, Endelman L, Sekar P. Disseminated histoplasmosis presenting as bilateral lower extremity paresis. Int J Infect Dis. 2020. 95: 265-7
9. Tai YF, Kullmann DM, Howard RS, Scott GM, Hirsch NP, Revesz T. Central nervous system histoplasmosis in an immunocompetent patient. J Neurol. 2010. 257: 1931-3
10. Voelker JL, Muller J, Worth RM. Intramedullary spinal Histoplasma granuloma. Case report. J Neurosurg. 1989. 70: 959-61
11. Wheat J, Myint T, Guo Y, Kemmer P, Hage C, Terry C. Central nervous system histoplasmosis: Multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine (Baltimore). 2018. 97: e0245