- Lillian S. Wells Department of Neurosurgery, University of Florida, Gainesville, Florida, United States,
- Department of Neuropathology, Mayo Clinic, Rochester, Minnesota, United States.
- Department of Pathology, University of Florida, Gainesville, United States.
- Department of Neurosurgery, Mayo Clinic, Jacksonville, Florida, United States.
DOI:10.25259/SNI_113_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel Louis Malnik1, Rachel Freedman Moor1, David Shin1, Dimitri Laurent1, Jorge Trejo-Lopez2, William Dodd1, Anthony Yachnis3, Ashley P. Ghiaseddin1, W. Christopher Fox4, Steven Roper1. Inflammatory myofibroblastic tumor masquerading as an anterior choroidal artery fusiform aneurysm. 21-Jun-2021;12:297
How to cite this URL: Samuel Louis Malnik1, Rachel Freedman Moor1, David Shin1, Dimitri Laurent1, Jorge Trejo-Lopez2, William Dodd1, Anthony Yachnis3, Ashley P. Ghiaseddin1, W. Christopher Fox4, Steven Roper1. Inflammatory myofibroblastic tumor masquerading as an anterior choroidal artery fusiform aneurysm. 21-Jun-2021;12:297. Available from: https://surgicalneurologyint.com/surgicalint-articles/10901/
Abstract
Background: Inflammatory myofibroblastic tumor is a rare, poorly understood tumor that has been found to occur in almost every organ tissue. Its location within the central nervous system is uncommon, and patients tend to present with nonspecific symptoms.
Case Description: A female in her eighth decade presented to neurosurgery clinic with complaints of headache and dizziness. Initial imaging was consistent with a low-grade, benign brain lesion in the region of the left choroidal fissure. She was recommended for observation but returned 1 month later with progressive symptoms and doubling of the lesion size. She underwent surgical resection and was found to have an IMT arising from the wall of the left anterior choroidal artery.
Conclusion: Intracranial IMT remains a rare and poorly understood entity. The present case demonstrates a novel presentation of IMT in an adult patient and exemplifies the heterogeneity of the disease presentation.
Keywords: Aneurysm, Anterior choroidal, Inflammatory myofibroblastic tumor, Spindle cell tumor
INTRODUCTION
Inflammatory myofibroblastic tumors (IMTs) comprise a spectrum of enigmatic lesions that occur in almost all organ systems, but rarely involve the central nervous system (CNS).[
CASE REPORT
History
A female in her eighth decade presented to the neurosurgery clinic complaining of 1 week dizziness and right-sided weakness. Neurologic examination was normal. MRI brain demonstrated a contrast-enhancing lesion centered at the left choroidal fissure between the amygdala and cerebral peduncle [
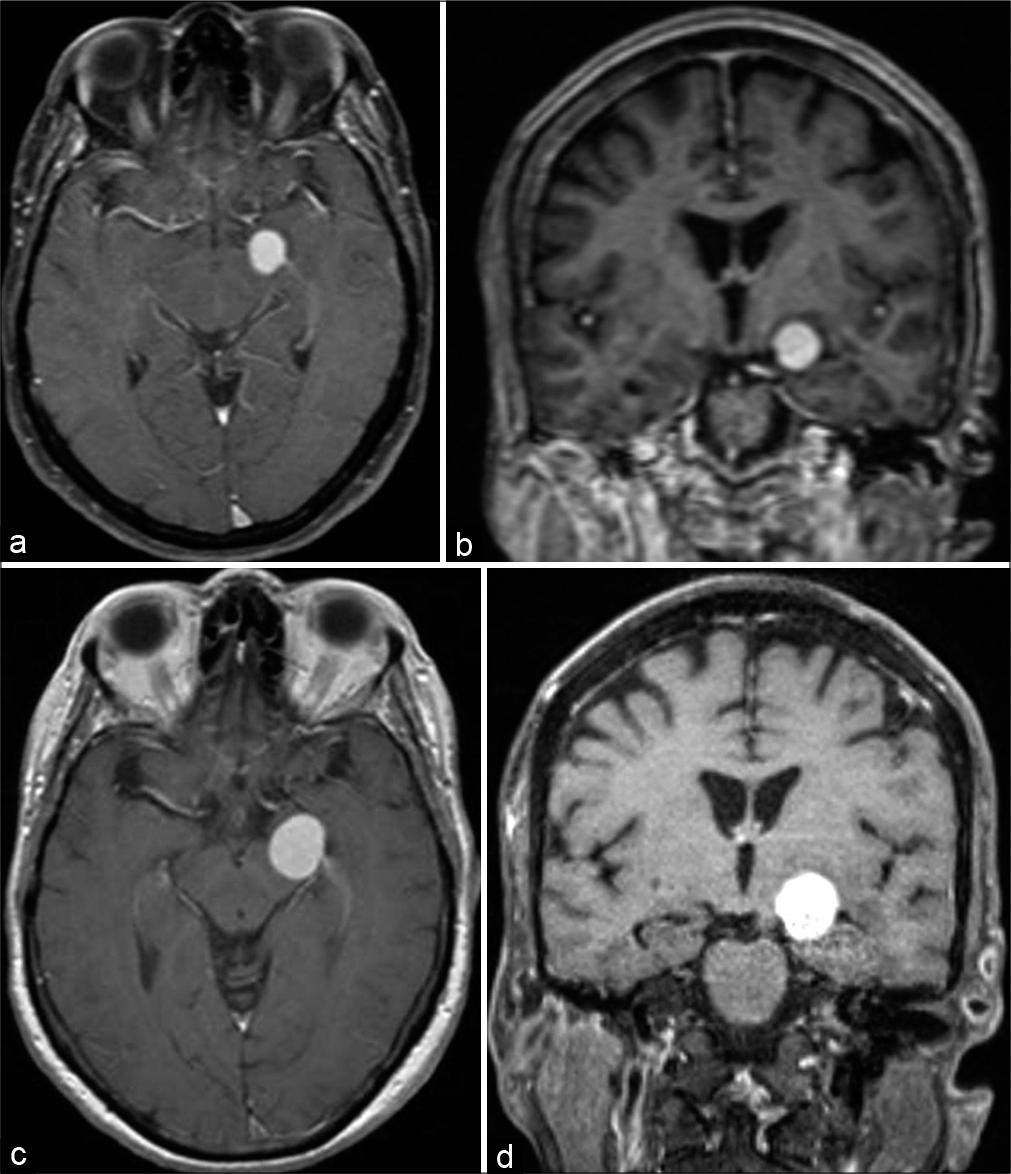
Figure 1:
(a) Axial plane contrast-enhanced MRI demonstrating lesion at time of initial presentation. (b) Coronal plane contrast-enhanced MRI demonstrating lesion at time of initial presentation. (c) Axial plane contrast-enhanced MRI demonstrating interval growth 1 month after initial presentation. (d) Coronal plane contrast-enhanced MRI demonstrating interval growth 1 month after initial presentation.
Operation
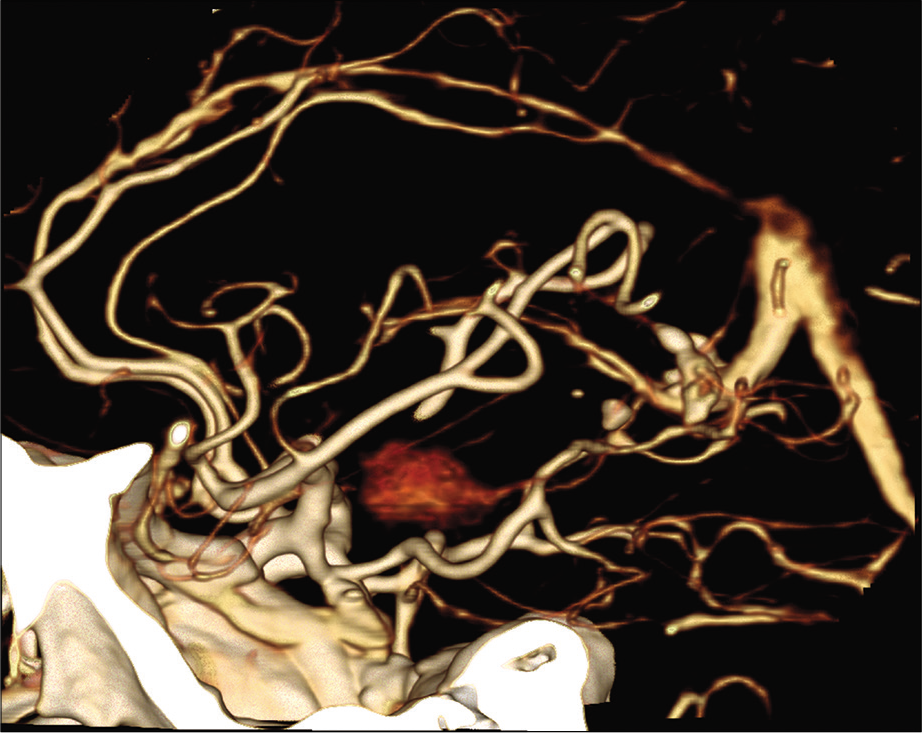
The patient was placed under general anesthesia in a Mayfield head holder with the head rotated at 45 degree angle to the right with a bump placed under the left shoulder. A left frontotemporal craniotomy was performed in standard fashion. Using frameless stereotactic guidance, we made a corticectomy in the anterior portion of the superior temporal gyrus. Brain tissue was removed along the inferior bank of the Sylvian fissure down to the region of the amygdala and the anterior portion of the temporal horn. We identified a discrete mass medial to the uncus that was firm and somewhat vascular. Initial biopsy demonstrated inflammatory tissue. After circumferential dissection, the mass was found to be clearly arising from the anterior choroidal artery and there was concern that the lesion represented a thrombosed fusiform aneurysm. Vascular neurosurgery was invited to participate in further surgical management of the lesion. Repeat biopsy demonstrated inflammatory and smooth muscle cells, suggesting sampling of the tunica media of a vascular lesion. Micro-Doppler was performed and demonstrated patency of the left posterior communicating artery and no flow within the left anterior choroidal artery. It was felt safe to sacrifice the anterior choroidal artery without causing harm. A straight Yasargil aneurysm clip was applied to the anterior choroidal artery proximal to the lesion. Circumferential microdissection of the lesion was carried out. The artery was then sectioned and the mass was sent for permanent pathology as a single specimen. Hemostasis was then obtained, and the wound was closed in usual sterile fashion.
Pathological findings
Gross examination of the tumor revealed a red-brown, ovoid portion of tissue with apparent encapsulation by a thin fibrous layer. Serial sections of the specimen revealed tan-white, partially hemorrhagic soft cut surfaces.
Microscopic examination of the tumor with hematoxylin and eosin stained sections demonstrated a proliferation of spindle cells arranged in short fascicles, with a prominent background of acute and chronic inflammation [
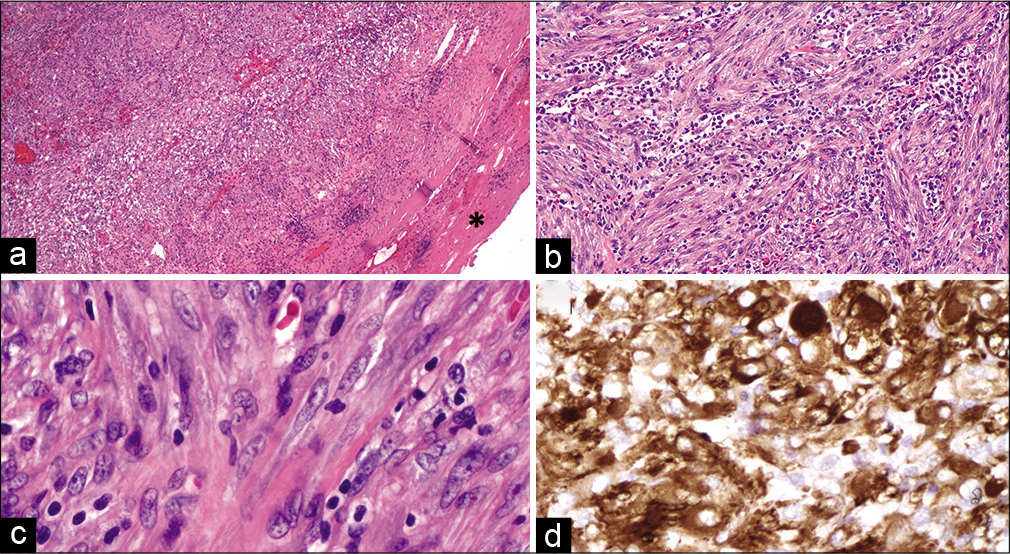
Figure 3:
Inflammatory myofibroblastic tumor. Examination of the tumor on low magnification revealed a fairly well-demarcated cellular proliferation, demonstrating an incomplete fibrous pseudocapsule on the periphery (a, asterisk, ×5). The tumor was composed of spindled cells arranged in short fascicles with a background of prominent, multifocal acute and chronic inflammation, as well as frequent blood vessels (b, ×20). Tumor cells showed elongated, monotonous nuclei with small nucleoli (c, ×40) and were diffusely and strongly reactive for smooth muscle actin immunohistochemistry (d, ×40). (a-c) Hematoxylin and eosin. (d) Smooth muscle actin immunohistochemistry.
A representative formalin-fixed, paraffin-embedded tissue block of tumor was macrodissected for RNA extraction, which was directed toward sequence using the Archer® FusionPlex® Sarcoma kit from ArcherDx on the Illumina NextSeq instrument to high uniform depth. Sequence data were processed using the kit manufacturer’s analysis pipeline (Archer Analysis v5.1.3) designed to accurately detect gene fusions. Per sequencing study, no pathological fusions were detected.
Postoperative course
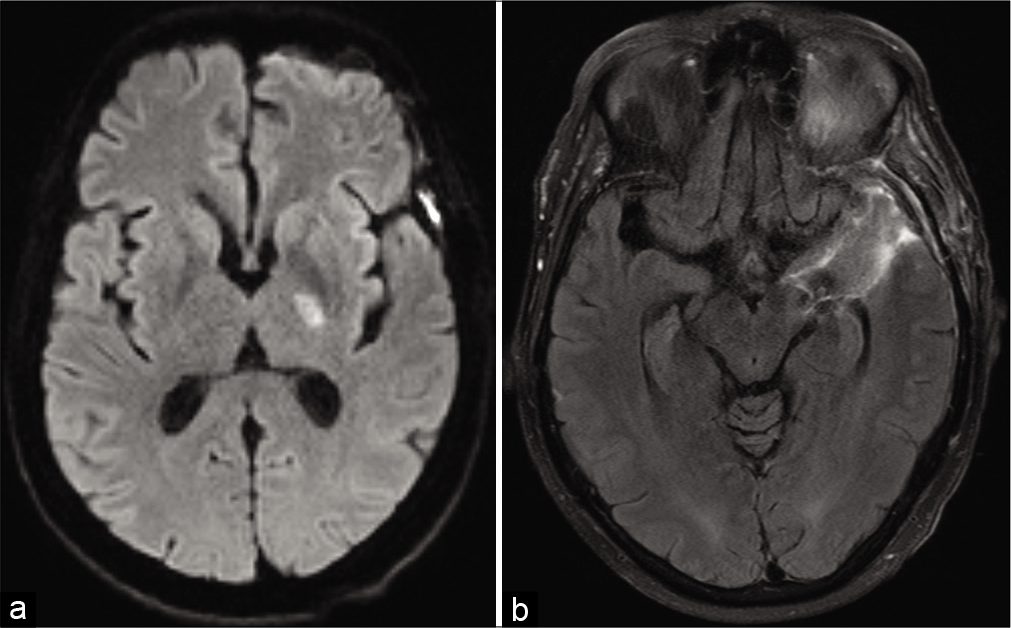
Postoperatively, she developed right upper extremity weakness. MRI demonstrated a small infarct of the left posterior limb of the internal capsule consistent with an anterior choroidal artery infarct [
DISCUSSION
IMTs constitute a rare group of heterogeneous lesions characterized histologically by myofibroblastic spindle cells mixed with a polyclonal infiltrate of plasma cells, lymphocytes, and eosinophils.[
IMT involvement of the CNS (IMT-CNS) is a rare occurrence, with a total number of sporadic IMT-CNS reported of less than 100.[
The present case is an illustrative example of the difficulty in diagnosing and treating IMT-CNS. Although imaging characteristics favored meningioma, rapid growth over short interval was inconsistent with the benign appearance of the WHO Grade I meningioma. Accelerated growth has been reported of pulmonary IMT in the setting of corticosteroid therapy. The precise mechanism is unclear, but has been postulated to be related to cellular proliferation due to immunosuppression.[
CONCLUSION
Intracranial inflammatory myeofibroblastic tumor remains a rare and poorly understood entity. Radiographic diagnosis is difficult, as the IMT appearance mimics more common CNS pathology. The present case demonstrates a novel presentation of IMT in an adult patient and exemplifies the heterogeneity of the disease presentation.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010. 363: 1727-33
2. Carswell C, Chataway J. The successful long-term management of an intracranial inflammatory myofibroblastic tumor with corticosteroids. Clin Neurol Neurosurg. 2012. 114: 77-9
3. Coffin CM, Dehner LP, Meis-Kindblom JM. Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: An historical review with differential diagnostic considerations. Semin Diagn Pathol. 1998. 15: 102-10
4. Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: A clinical and pathological survey. Semin Diagn Pathol. 1998. 15: 85-101
5. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19: 859-72
6. Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: A comparative immunohistochemical study. Am J Surg Pathol. 2001. 25: 1364-71
7. Denis DJ, Elayoubi K, Weil AG, Berthelet F, Bojanowski MW. Inflammatory myofibroblastic tumors of the central nervous system that express anaplastic lymphoma kinase have a high recurrence rate. Surg Neurol Int. 2013. 4: 70
8. Fletcher CD, Krishnan UK, Mertens F.editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. WHO Classification of Tumours. Lyon, France: IARC Press; 2002. p.
9. Häusler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B. Inflammatory pseudotumors of the central nervous system: Report of 3 cases and a literature review. Hum Pathol. 2003. 34: 253-62
10. Jeon YK, Chang KH, Suh YL, Jung HW, Park SH. Inflammatory myofibroblastic tumor of the central nervous system: Clinicopathologic analysis of 10 cases. J Neuropathol Exp Neurol. 2005. 64: 254-9
11. Kato K, Moteki Y, Nakagawa M, Kadoyama S, Ujiie H. Inflammatory myofibroblastic tumor of the cerebellar hemisphere-case report. Neurol Med Chir (Tokyo). 2011. 51: 79-81
12. Kim JH, Chang KH, Na DG, Park SH, Kim E, Han DH. Imaging features of meningeal inflammatory myofibroblastic tumor. AJNR Am J Neuroradiol. 2009. 30: 1261-7
13. Konishi H, Ookuma H, Hara H, Okayasu H, Wakayama Y, Saiki S. A case of plasma cell granuloma associated with cerebral infarction. Rinsho Shinkeigaku. 1996. 36: 854-7
14. Lata KA, Upadhyay V, Pawar SS. A rare case of inflammatory myofibroblastic tumor of meninges. J Pediatr Neurosci. 2018. 13: 255-9
15. Lee MH, Lee HB, Lee YC, Rhee YK, Lee EJ, Chung MJ. Bilateral multiple inflammatory myofibroblastic tumors of the lung successfully treated with corticosteroids. Lung. 2011. 189: 433-5
16. Nagumo Y, Maejima A, Toyoshima Y, Komiyama M, Yonemori K, Yoshida A. Neoadjuvant crizotinib in ALK-rearranged inflammatory myofibroblastic tumor of the urinary bladder: A case report. Int J Surg Case Rep. 2018. 48: 1-4
17. Nishino T, Toda J, Nakatsuka T, Kimura T, Inaoka T, Terada H. IgG4-related inflammatory pseudotumors mimicking multiple meningiomas. Jpn J Radiol. 2013. 31: 405-7
18. Panigada S, Sacco O, Girosi D, Magnano GM, Tuo P, Tarantino V. Corticosteroids may favor proliferation of thoracic inflammatory myofibroblastic tumors. Pediatr Pulmonol. 2014. 49: E109-11
19. Park JH, Yoon WS, Chung DS. Unusual radiologic finding of intracranial inflammatory myofibroblastic tumor presenting a cyst with mural nodule. Brain Tumor Res Treat. 2015. 3: 138-40
20. Sahu A, Prabhash K, Noronha V, Joshi A, Desai S. Crizotinib: A comprehensive review. South Asian J Cancer. 2013. 2: 91-7
21. Sebire NJ, Ramsay A, Sheppard M, Malone M, Harding B, Risdon RA. Intravascular inflammatory myofibroblastic tumors in infancy. Pediatr Dev Pathol. 2002. 5: 400-4
22. Singhal AB, Gonzalez RG, Chwalisz BK, Mukerji SS. Case 26-2020: A 60-Year-old woman with altered mental status and weakness on the left side. N Engl J Med. 2020. 383: 764-73
23. Swain RS, Tihan T, Horvai AE, di Vizio D, Loda M, Burger PC. Inflammatory myofibroblastic tumor of the central nervous system and its relationship to inflammatory pseudotumor. Hum Pathol. 2008. 39: 410-9
24. Wang X, Chen Y, Wu X, Zhang H. Intracranial inflammatory myofibroblastic tumor with negative expression of anaplastic lymphoma kinase: A case report and review of the literature. World Neurosurg. 2019. 125: 117-22
25. Yang L, Li W, Zhang H. Inflammatory myofibroblastic tumor of carotid artery resulting in recurrent syncope: A case report. Head Neck. 2016. 38: E2461-3