- Department of Neurosurgery, University of Florida, Gainesville, Florida, United States.
- Department of Neurosurgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States.
Correspondence Address:
Heather Pinckard-Dover
Department of Neurosurgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas, United States.
DOI:10.25259/SNI_763_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Heather Pinckard-Dover1, Hytham Al-Hindi2, Grace Goode2, Hayden Scott2, Erika Petersen2. Influence of stereotactic imaging on operative time in deep brain stimulation. 02-Mar-2021;12:82
How to cite this URL: Heather Pinckard-Dover1, Hytham Al-Hindi2, Grace Goode2, Hayden Scott2, Erika Petersen2. Influence of stereotactic imaging on operative time in deep brain stimulation. 02-Mar-2021;12:82. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10617
Abstract
Background: Various techniques are used across institutions for implantation of deep brain stimulation (DBS) leads. The most used techniques for each step include preoperative MRI fused to in-frame CT, intraoperative fluoroscopy, and postoperative CT, but postimplantation MRI also is used, as it was at our center. We present the quality assurance study performed at our institution after a change from postimplantation MRI performed across the hospital to postimplantation in room CT.
Methods: Retrospective chart review of 123 patients who underwent bilateral DBS leads placement without same-day generator placement that was performed. The patients were divided by the type of postoperative imaging that was obtained. Patients were excluded if a unilateral lead placement was performed, if the case was a revision of an existing lead or deviated from the normal protocol. Operative room times and procedure times for each group were analyzed with Wilcoxon rank sums test (WRST) to determine any significant differences between groups.
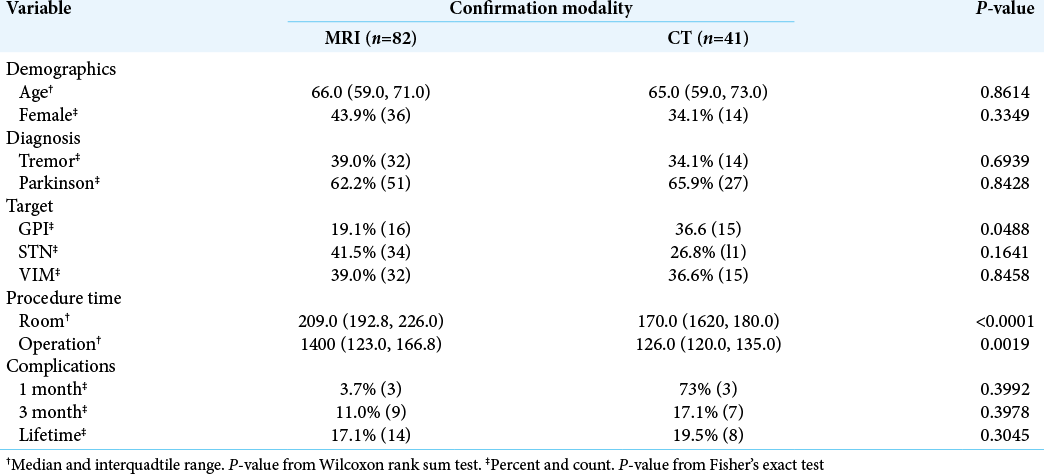
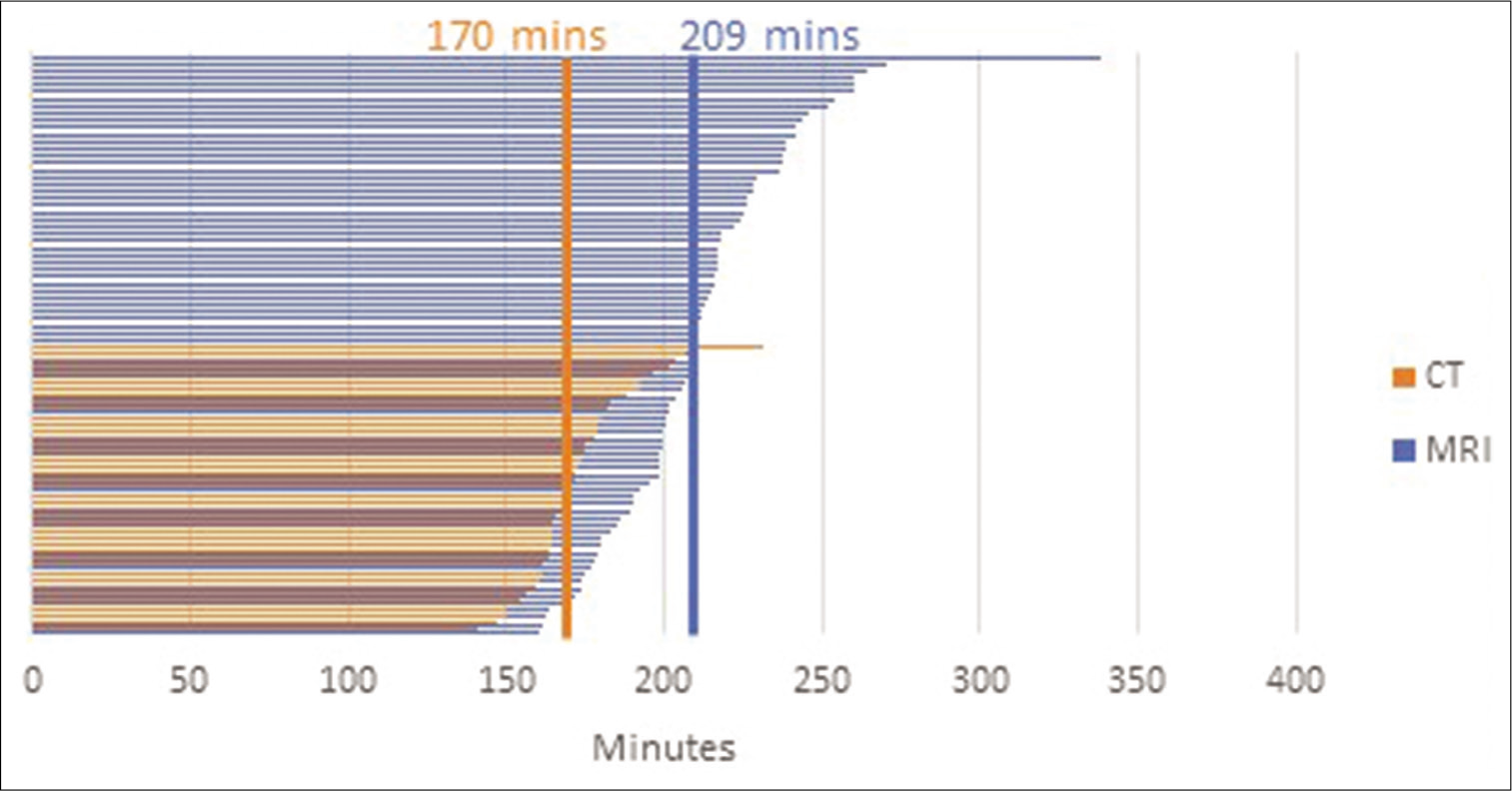
Results: Postoperative MRI was performed for 82 patients, while postoperative CT was performed for 41 patients. A WRST showed a significant reduction in both operative room time (209 min to 170 min, P P = 0.0019).
Conclusion: In-room CT allowed for a significant reduction in operative room time. Lower operative room time has been associated with increased patient comfort, and decreased cost. CT did not alter the revision rate for procedures. The significant reduction in procedure time may be attributed to increased team familiarity with procedure over time.
Keywords: Deep brain stimulation, Operative time, Stereotactic imaging
INTRODUCTION
Deep brain stimulation (DBS), a common neurosurgical procedure that has been in practice since the 1980s, is commonly used to treat diseases such as Parkinson’s disease (PD), essential tremor (ET), and dystonia. Innovations in surgical technology, including improvements in neuroimaging and stereotactic navigation, have led to a diversity of methodology for implantation. Surgeons vary in their preferred technique for implantation of DBS systems. An international survey in 2013 indicated that a stereotactic approach using preoperative MRI fused with CT in-frame, intraoperative fluoroscopy, and postoperative CT was the most frequently used technique.[
In 2017, in efforts to increase patient comfort and workflow efficiency, a change was made to the standard DBS operation at our institution. As with all changes, quality assurance studies should be performed along the way to verify the technique provides the desired effects without causing unexpected complications.
MATERIALS AND METHODS
Patients at the University of Arkansas for Medical Sciences implanted with DBS with neurosurgeon EP underwent a two-stage procedure with awake, frame-based intracranial lead placement in stage 1 and implanted pulse generator placement in the second stage generally 7 days later. Procedures were similar for all indications and targets. The faculty surgeon was assisted by rotating resident surgeons during all cases. For intracranial lead placement, the patient arrived in the preoperative suite early on the morning of their operation. While in the preoperative suite, a Leksell (Elekta, Stockholm, Sweden) stereotactic frame was applied under local anesthetic. The patient was transported to the MRI suite for stereotactic image acquisition. The patient returned to the preoperative suite, while the neurosurgeon performed stereotactic planning. Once frame-based coordinates were obtained, the patient was brought into the operating room and positioned supine on the operative table. The frame was set to the first side coordinates. The patient was prepped and draped in sterile fashion and local anesthetic infiltrated at the incision sites. The incision was made, burr hole drilled, and outer ring of the burr hole fixation device placed in usual fashion. An impedance electrode was passed to target checking impedances along the planned trajectory. The lead was advanced to target. Fibrin sealant was inserted into the burr hole and the center locking mechanism of burr hole fixation device used to secure the lead. The lead was connected to a macrostimulation platform. The neurosurgeon then proceeded to the side of the patient to perform intraoperative testing. The lead was tested with a 0–3+, 60 mcs, 130 Hz configuration. The patient was tested just after implantation, and at increments of 0.5 V or smaller until clinical side effect was appreciated or until 5 V was reached. If testing reached clinical side effect at too low an amplitude, the lead was repositioned to a more appropriate target and macrostimulation repeated. Once the lead was in desired position, a fluoroscopic image was taken, the stage disassembled and another fluoroscopic image obtained to ensure the lead did not migrate. The frame was then positioned to the coordinates for the contralateral side, where the process was repeated. Once both leads were in place, proximal lead boots were placed and the two proximal leads tunneled under the galea to the parietal region of the desired side. The incisions were irrigated well and closed. The frame was released from the bed and the patient transferred to a stretcher. In the surgical protocol for cases before 2017, the patient was transferred across the hospital to the MRI scanner, where a stereotactic image was obtained. Once the leads were verified to be in desirable location, the operative room was called and the staff allowed to break down the field. In the event, the lead was not in a good position, the patient was transported back across the hospital to the operating room and the lead revised.
In 2017, the postimplantation imaging protocol changed with the acquisition of an intraoperative CT scanner. Instead of transporting the patient across the hospital for a stereotactic MRI, the patient’s head contained in the Leksell frame was detached from the bed, the regular head extension of the operative table was attached to support the patient and the Mayfield frame removed from the bed. The intraoperative mobile CT scanner was positioned over the patient. The CT localizer box was attached to the frame and a stereotactic CT obtained. Lead position was checked against the planned trajectories, and if the leads were in a desirable place the frame removed and the patient transported to the recovery room. In the event, the leads were not in a desirable place, the head extension of the bed was removed, Mayfield frame reattached, and the lead revised.
In December 2018, we performed a quality assurance study to ensure that the new technique was providing the desired benefit of efficiency without any unwanted complications and used SQUIRE guidelines to report our findings.[
Patients were divided into two main groups based on preand postoperative imaging modalities: MRI-verified and CT-verified. A total of 123 patient cases were reviewed after exclusion of unilateral cases, cases where generator and extension placement were completed during the same procedure, lead revisions, and a case where revision was required after MRI showed a misplaced lead, due to the expected significant variance in operative times in these instances. For purposes of this study, the operative room time was recorded in minutes from the time the patient entered the room until the operative room staff was released to start cleaning the room. The procedure time was recorded in minutes from the time of incision to the time of closure. Wilcoxon rank sum tests were used to compare the imaging modality groups with respect to operative room time and procedure time. Due to the similarity in procedure between targets and diseases and to maintain adequate statistical power, no sub-analysis was done based on target or disease.
RESULTS
Of the 123 patients meeting inclusion criteria in the 54-month study period, postoperative MRI was obtained for 82 patients and postoperative CT for 41 patients. There were no statistical differences in sex or age between the groups [
DISCUSSION
Operative technique for placement of DBS systems varies greatly between institutions. Many of these techniques will result in similar procedure accuracy, safety, and clinical outcomes. Nuances of procedures have been debated at length.[
Our data reveal that the change in lead verification procedure has meaningfully shortened operative room time and procedure time. There was a significant reduction in operative room time (209 min to 170 min, P < 0.0001). The procedure time reduction (140 min to 126 min, P = 0.0019) is likely a result of improved efficiency as operative room staff gained familiarity with the procedure over time, since there was not a change to the overall surgical technique. There remains a 25 min difference between reduction in procedure time and operative room time that we believe is a direct result of the change in lead verification procedure related to transport and imaging time. No direct comparison of patient comfort can be made based on the retrospective data set; however, one can surmise that shorter operative room time in an awake patient without transport across the hospital would be more comfortable. Operative room and anesthesia staff members have often expressed to the neurosurgeon their preference for the in-room CT over transportation to MRI.
In adopting any new technique, careful evaluation of changes in patient quality and outcomes should be assessed. The current quality assurance study was performed to assess the benefit in efficiency from our postimplantation imaging change as well as relieve concerns over the change in imaging modality and information yield. We were reassured that lead visualization and intracranial hemorrhage concerns were not impacted with the use of CT. Lead accuracy was not different in the two populations. We had expected that the reduction in transport across the hospital with fresh incisions and shorter operative time might decrease the long-term complication rate, but this did not significantly change (17.1% vs. 19.5%, P = 0.8045). The smaller size of the CT-imaged population did not statistically impact this analysis. The natural history and lifetime failure rate for DBS, including infection and mechanical complications in our series, are slightly higher than for other series.[
Reduction in operative times may translate into improved operative room efficiency and utilization. In some institutional settings, this may realize financial savings related to lower case time costs. More likely, the shorter DBS operative time could contribute a revenue benefit to the institution through the opportunity to increase operative case volume. The improvement of comfort of the experience to patients and the diminished challenges to the operating room staff and clinician team through eliminated transport across the hospital complex cannot be quantified using the current retrospective analysis, but these elements are important quality improvements. Our analysis confirmed that the reduction in operative room time is worth the change in technique, enabling improved utilization of the operative room, and staff resources. This is a quality assurance study evaluating the effects of a change in operative procedure at a single institution. There is no blinding and potentially a Hawthorne effect might bias the data; although our retrospective study was conceived after CT had been in use for over a year, the surgical team might have altered behavior in some of the latter cases in the series based on awareness of the study. We recognize that it is difficult to generalize the findings of our study to other institutions. We encourage other institutions who may be contemplating similar changes to conduct a quality assurance study that will like ours validate their efforts.
CONCLUSION
In-room CT has proven to be a useful tool in reducing operative room time for placement of bilateral DBS leads. Although the image information obtained is less detailed than with MRI, the verification of lead placement is accurate. The change in imaging technique does not appear to affect the overall complication rate. Reductions in operative times associated with the optimal lead-verification imaging techniques may help neurosurgical teams develop a more efficient operative plan for patients who undergo DBS, resulting in financial benefit to the institution, improved patient comfort, and clinician satisfaction.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Erika Petersen receives consulting and speaking fees from Medtronic, Neuros Medical, Nevro, and Abbott. She also receives research funding from Medtronic, Neuros Medical, ReNeuron, and Nevro. She has stock options in SynerFuse. None of these had any bearing on this manuscript.
References
1. Abosch A, Timmermann L, Bartley S, Rietkerek HG, Whiting D, Conolly PJ. An international survey of deep brain stimulation procedural steps. Stereotact Funct Neurosurg. 2013. 91: 1-11
2. Bullard AJ, Hutchison BC, Lee J, Chestek CA, Patil PG. Estimating risk for future intracranial, fully implanted, modular neuroprosthetic systems: A systematic review of hardware complications in clinical deep brain stimulation and experimental human intracortical arrays. Neuromodulation. 2019. 23: 411-26
3. Burchiel KJ, Mcartney S, Lee A, Raslan AM. Accuracy of deep brain stimulation electrode placement using intraoperative computed tomography without microelectrode recording. J Neurosurg. 2013. 119: 301-6
4. Danks RA, Rogers M, Aglio LS, Gugino LD, Black PM. Patient tolerance of craniotomy performed with the patient under local anesthesia and monitored conscious sedation. Neurosurgery. 1998. 42: 28-36
5. Foltynie T, Zrinzo L, Martinez-Torres I, Tripoliti E, Petersen E, Holl E. MRI-guided STN DBS in Parkinson’s disease without microelectrode recording: Efficacy and safety. J Neurol Neurosurg Psychiatry. 2011. 82: 358-63
6. Han C, Song Q, Ren Y, Luo J, Jiang X, Hu D. Dose-response association of operative time and surgical site infection in neurosurgery patients: A systematic review and meta-analysis. Am J Infect Control. 2019. 47: 1393-6
7. Mirzadeh Z, Chen T, Chapple KM, Lambert M, Karis JP, Dhall R. Procedural variables influencing stereotactic accuracy and efficiency in deep brain stimulation surgery. Oper Neurosurg (Hagerstown). 2019. 17: 70-8
8. Ogrinc G, Davies L, Goodman D, Batalden PB, Davidoff F, Stevens D. SQUIRE 2.0 (standards For quality improvement reporting excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016. 25: 986-92
9. Saleh C, Dooms G, Berthold C, Hertel F. Post-operative imaging in deep brain stimulation: A controversial issue. Neuroradiol J. 2016. 29: 244-9
10. Sorar M, Hanalioglu S, Kocer B, Eser MT, Comoglu SS, Kertmen H. Experience reduces surgical and hardware-related complications of deep brain stimulation surgery: A single-center study of 181 patients operated in six years. Parkinsons Dis. 2018. 2018: 3056018
11. Teton ZE, Blatt D, AlBakry A, Obayashi J, Ozturk G, Hamzaoglu V. Natural history of neuromodulation devices and therapies: A patient-centered survival analysis. J Neurosurg. 2019. 132: 1385-91
12. Teton ZE, Blatt D, AlBakry A, Obayashi J, Ozturk G, Hamzaoglu V. Natural history of neuromodulation devices and therapies: A patient-centered survival analysis. J Neurosurg. 2019. 132: 1385-91