- Clinical Neuroscience, Karolinska Institute, İstanbul, Turkey,
- Department of Medical Radiation Physics and Nuclear Medicine, Karolinska University Hospital, İstanbul, Turkey,
- Department of Neurosurgery, Beykoz Institute of Life Science and Biotechnology, Bezmialem Vakif University, İstanbul, Turkey,
- Department of Oncology, Royal Berkshire NHS Foundation Trust, Reading, Berkshire,
- Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden.
Correspondence Address:
Georges Sinclair
Department of Neurosurgery, Beykoz Institute of Life Science and Biotechnology, Bezmialem Vakif University, İstanbul, Turkey,
Department of Oncology, Royal Berkshire NHS Foundation Trust, Reading, Berkshire,
Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden.
DOI:10.25259/SNI_406_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Mominul Islam, Gerald Cooray, Hamza Benmakhlouf, Mustafa Hatiboglu, Georges Sinclair. Integrating navigated transcranial magnetic stimulation motor mapping in hypofractionated and single-dose gamma knife radiosurgery: A two-patient case series and a review of literature. 28-Feb-2020;11:29
How to cite this URL: Mominul Islam, Gerald Cooray, Hamza Benmakhlouf, Mustafa Hatiboglu, Georges Sinclair. Integrating navigated transcranial magnetic stimulation motor mapping in hypofractionated and single-dose gamma knife radiosurgery: A two-patient case series and a review of literature. 28-Feb-2020;11:29. Available from: https://surgicalneurologyint.com/surgicalint-articles/9879/
Abstract
Background:The aim of the study was to demonstrate the feasibility of integrating navigated transcranial magnetic stimulation (nTMS) in preoperative gamma knife radiosurgery (GKRS) planning of motor eloquent brain tumors.
Case Description:The first case was a 53-year-old female patient with metastatic breast cancer who developed focal epileptic seizures and weakness of the left hand. The magnetic resonance imaging (MRI) scan demonstrated a 30 mm metastasis neighboring the right precentral gyrus and central sulcus. The lesion was treated with adaptive hypofractionated GKRS following preoperative nTMS-based motor mapping. Subsequent follow-up imaging (up to 12 months) revealed next to complete tumor ablation without toxicity. The second case involved a previously healthy 73-year-old male who similarly developed new left-handed weakness. A subsequent MRI demonstrated a 26 mm metastatic lesion, located in the right postcentral gyrus and 5 mm from the hand motor area. The extracranial screening revealed a likely primary lung adenocarcinoma. The patient underwent preoperative nTMS motor mapping prior to treatment. Perilesional edema was noted 6 months postradiosurgery; nevertheless, long- term tumor control was demonstrated. Both patients experienced motor function normalization shortly after treatment, continuing to final follow-up.
Conclusion:Integrating preoperative nTMS motor mapping in treatment planning allowed us to reduce dose distributions to perilesional motor fibers while achieving salvage of motor function, lasting seizure freedom, and tumor control. These initial data along with our review of the available literature suggest that nTMS can be of significant assistance in brain radiosurgery. Prospective studies including larger number of patients are still warranted.
Keywords: Adaptive hypofractionated gamma knife radiosurgery, Karnofsky performance scale, Magnetic resonance imaging, Motor region, Single-dose gamma knife radiosurgery, Transcranial magnetic stimulation
INTRODUCTION
The surgical management of metastatic lesions in the eloquent brain remains complex in terms of tumor control and preservation of neurologic function, particularly in cortical motor areas where functional relevance is difficult to predict purely from anatomical imaging studies. The application of presurgical functional mapping with the aim of modifying microsurgery planning and reduce postoperative functional deficits is now a common procedure; the unique meta- analysis by Raffa et al. provides further support to the latter concept and remains to date an unequivocal landmark of reference.[
MATERIALS AND METHODS
Inclusion criteria
Subject to multidisciplinary discussion, as well as institutional and ethical approval, two adult patients with MRI-verified metastatic lesions in or adjacent to cortical motor areas were recruited for the purpose of this study. In both cases, microsurgery was deemed less suitable than radiosurgery due to topoanatomical limitations and the extension of extracranial metastatic disease; other forms of radiotherapy or systemic treatment were assessed as of no benefit to these patients. Patients were required to have a Karnofsky Performance scale (KPS) of at least 70 (recursive partitioning analysis [RPA] 1–2). Previous microsurgery, presence of perilesional edema (resistant to steroids or not) and extracranial tumor activity were not criteria for exclusion. However, future or ongoing oncological treatment and survival expectancy of at least 6 months were mandatory. In this particular setting, one patient was accepted for dose-volume adaptive hypofractionated GKRS while the other was planned for single-dose GKRS.
Pre- and post-GKRS nTMS protocol
Protocols for nTMS motor mapping followed updated institutional guidelines and previously published medical data.[
Complementary studies: pre- and post-GKRS electroencephalography (EEG)
Both patients were examined pre- and posttreatment with complementary scalp-EEG studies. Electrodes (21-scalp electrodes) were positioned using the 10–20 system; Fp1, Fp2, F7, F8, T3, T4, T5, T6, O1, O2, F3, F4, C3, C4, P3, P4, Fz, Cz, Pz, A1, and A2. Electrode impedances were kept below 5 kΩ. The recording consisted of 30 min of spontaneous activity with the patient awake in a supine position. The patients were provoked using 3 min of hyperventilation and photostimulation. The pre- and posttreatment EEGs showed no epileptiform activity; however, in Case 2, a focal slowing was demonstrated before and after the radiosurgery in the right central regions.
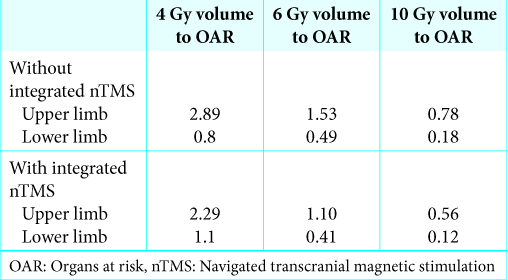
Integrating nTMS motor assessments to GKRS planning
Optimal cranium fixation was achieved mounting the stereotactic Leksell® frame. As a result, no margins were required outside the gross tumor volume limits. To attain ideal dynamic target definition, a stereotactic MRI was performed before each GKRS. MRI sequences included pre- and postgadolinium enhanced T1 fast spin echo, postgadolinium 3D T1-weighted volume FSPGR reconstructed in three planes as well as axial T2-weighted propeller, 1.5 T GE Discovery 450w. All positive nTMS points were fused together with the anatomical MRI obtaining identifiable motor points deemed as organs at risk (OAR) ultimately saved as a digital imaging and communication file. The data were then successfully imported to the LGP system and taken into consideration for GKRS planning. The corresponding GKRS treatment plan was set on the preoperative MRI evolution, and the presence of treatment feasibility variables described elsewhere.[
Case description
Case 1
A 53-year-old female patient with metastatic breast cancer, previously treated with both chemo- and immunotherapy, developed focal epileptic seizures involving her right arm. The investigative computed tomography (CT)-scan and MRI of the brain showed four intra-axial metastases, the largest (6.7 cc) located in the left precentral gyrus (M4). Extracranial CT screening showed stable appearances of disease, including her liver metastases. The patient was planned for adaptive hypofractionated GKRS of M4 and for single fraction GKRS treatment for the remaining lesions (M1-M3); due to their “safe” location, M1-M3 were excluded from this study. A preoperative nTMS for perilesional motor- mapping was performed the day before GKRS 1 (November 2016). In this case, a total of three muscles from the upper limb (adjacent to M4) were mapped and identified as OAR [
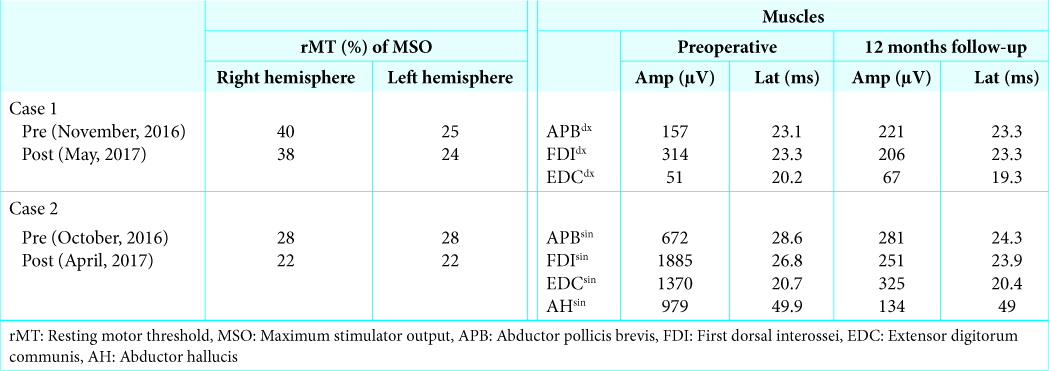
Figure 1:
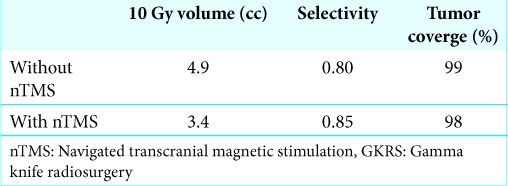
Structural image showing lesion and gamma knife radiosurgery (GKRS) planning in Case 1. (a) Yellow line = peripheral prescription isodose line. Outer and inner green lines = dose distribution inside and outside target. White rectangles = navigated transcranial magnetic stimulation (nTMS) motor points overlayed at 20–25 mm depth. (b) Magnetic resonance-images superimposed with pre-GKRS nTMS-determined organs at risk representing the arm and hand motor function areas for Case 1. Red = gross tumor volume (treatment target); Magenta = upper limb; Blue = lower limb.
Case 2
A previously healthy 73-year-old man developed gradual left- hand paresis (September 2016). A brain CT and subsequent MRI showed a 26 mm metastatic lesion in the right postcentral gyrus and postcentral sulcus, 5 mm from the hand motor cortex (M1), as well as an 18 mm metastasis (M2) located medially in the right superior frontal gyrus. Due to its location, M2 was deemed safe for GKRS and excluded from this study; both lesions had extensive perilesional edema. An investigative CT-scan of the thorax and abdomen revealed a 6 cm lung tumor in the right lower lobe as well as mediastinal lymph node infiltration. The histology of the primary was then confirmed to be a lung adenocarcinoma (October 2016). The patient was accepted for upfront GKRS management of M1 and M2 before starting systemic treatment. As in the first case, the patient underwent a preoperative nTMS for perilesional motor-mapping before starting treatment. Likely secondary to ongoing steroid treatment, the underlying nTMS MRI revealed a slight volume reduction of M1 (24 mm, vol =5 cc) while unchanged volumetry for M2 (1.3 cc). Due to the location of M1 in relation to the motor homunculus, three muscles from the upper limb and one lower limb muscle were mapped and identified as OAR [
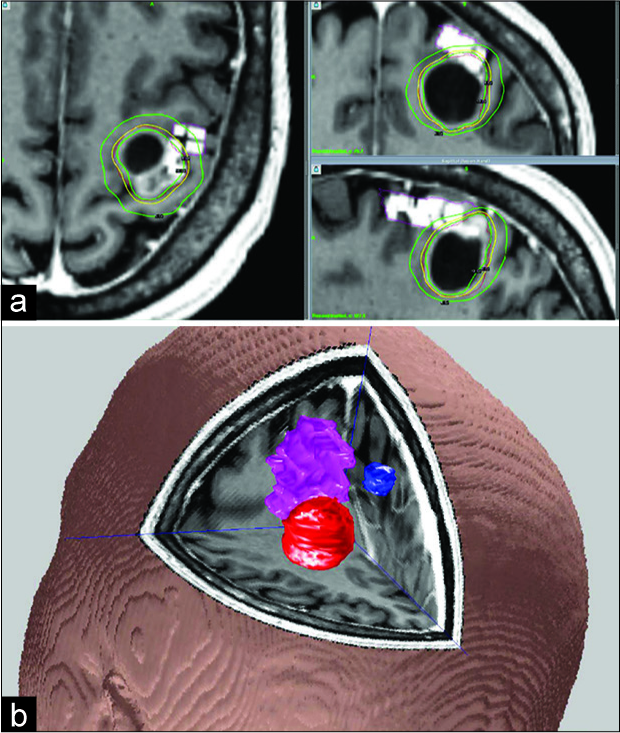
Figure 2:
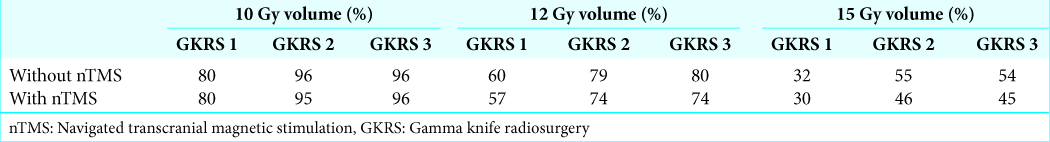
Structural image showing lesion and GKRS planning in Case 2. (a) Yellow line = peripheral prescription isodose line. Outer green line = dose distribution outside target (10 Gy-isodose line). White rectangles = navigated transcranial magnetic stimulation motor points overlayed at 20–25 mm depth. (b) Magnetic resonance-images superimposed with transcranial magnetic stimulation for Case 2. Gross tumor volumes in red (most anterior target included in the study) and organs at risk in magenta (upper limb) and blue (lower limb).
RESULTS
Case 1
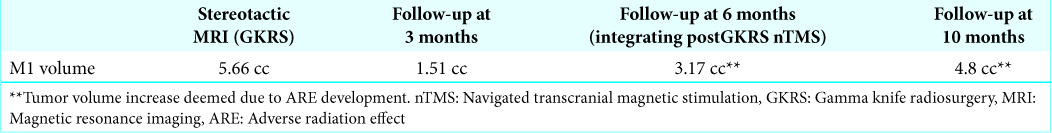
The treatment was well tolerated. M4 was 7.4 cc at GKRS 1; 16% of volume reduction was achieved between GKRS 1 and GKRS 3 [
Figure 3:
Tumor volume dynamics post-GKRS (see
Case 2
As in Case 1, the treatment was well tolerated. Follow-up MRI at 1 month showed slight volume reduction of M1 and M2 as well as reduced edema. The follow-up CT scan at 3 months showed further volume reduction of M1 and M2 but progression of the perilesional edema around M1 subsequently assessed as an ARE [
Figure 4:
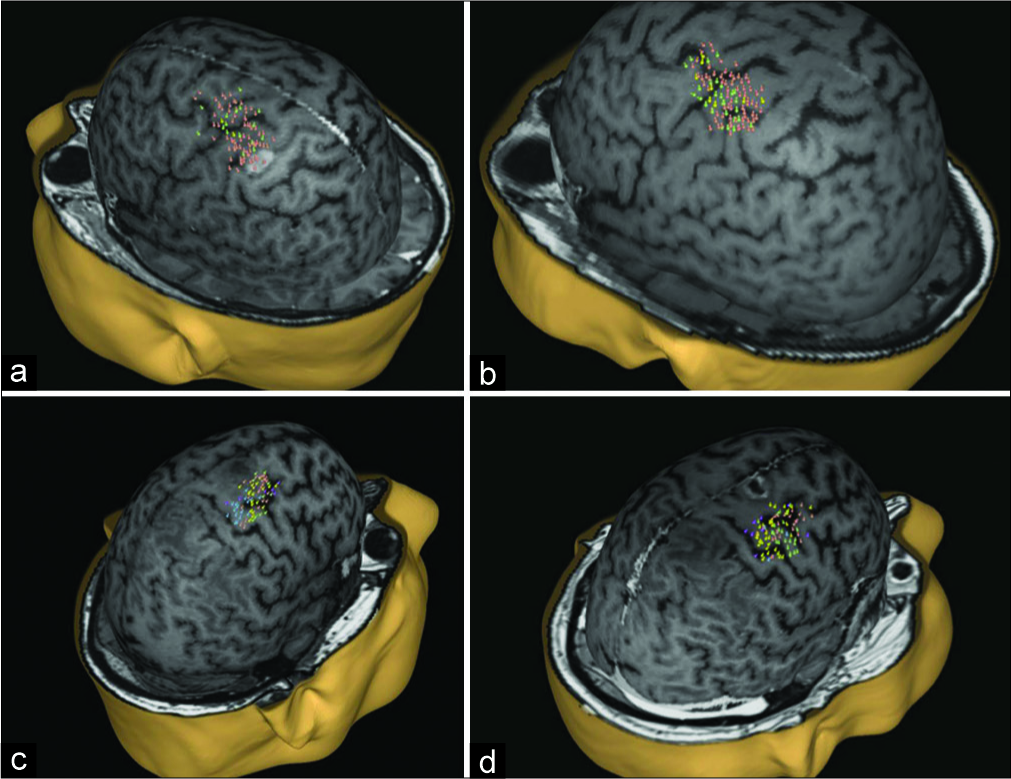
nTMS motor mapping – pre- and post-GKRS for Case 1 and 2. nTMS motor mapping of three upper limb muscles (mAPB - green, mFDI - orange, mEDC - yellow) contralateral to the side of the stimulation. (a) Case 1 pre-GKRS nTMS. (b) Case 1 – 12 months post-GKRS nTMS. (c) Case 2 pre-GKRS nTMS. (d) Case 2 – 6 months post-GKRS nTMS.
DISCUSSION
Rationale of the study
The present paper illustrates the complexity of metastatic brain disease in motor eloquent cortex. Patients with such lesions are generally at increased risk of postsurgical neurological deterioration with reduced gross and fine motor performance in the arm and hand. Furthermore, tumors located in the rolandic and perirolandic regions remain a source of concern due to the ensuing risk of developing epileptic seizures. These patients may benefit from focal therapy such as single fraction and hypofractionated GKRS;[
Nevertheless, the application of nTMS in radiosurgery is fairly new. Historically, the use of TMS in presurgical mapping remained limited until the availability of MRI- based neuronavigation assisted surgical series. In this context, intraoperative mapping of motor eloquent cortex under general anesthesia and speech mapping during awake surgery have generally been performed applying direct cortical stimulation (DCS); although this method is widely considered the gold standard of cortex functional mapping, its use in GKRS remains precluded by invasive requirements.
nTMS and radiosurgery: what is the evidence?
Functional mapping modalities, for example, functional MRI (fMRI),[
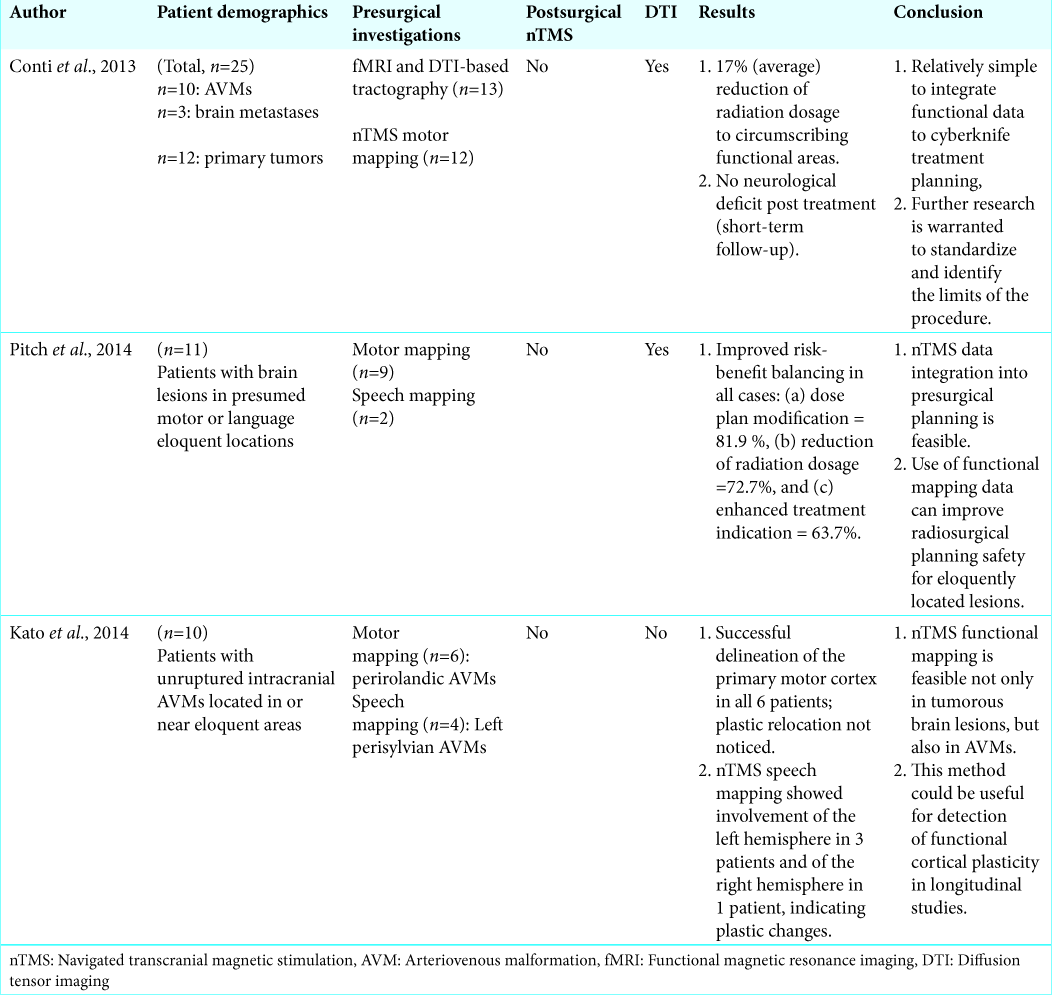
To further substantiate the above-mentioned presumptions, we feel worth discussing three pioneer studies applying nTMS alone in radiosurgical planning [
Further aspects
As reported in this case series, both patients had metastatic brain lesions assessed as radiosensitive, yet treated with two different GKRS modalities due to different tumor- surrogate and perilesional variables. Unlike previously published data,[
CONCLUSION
In our two patient case series, nTMS was safely and effectively integrated in single-dose and hypofractionated GKRS. Functional treatment planning based on nTMS estimates to the primary motor cortex resulted in less toxic dose distribution to the designated OAR in comparison to anatomical MRI-based GKRS planning. The latter was achieved without compromising intratumoral dose distribution. The relationship between the type of radiation schedule applied and possible subsequent long-term radiation-induced focal plastic distortions warrants further analysis. These initial data along with our review of the medical literature suggest that nTMS can be of valuable assistance in brain radiosurgery. Prospective studies involving larger, homogenous groups of patients are necessary to validate and better understand the clinical significance of nTMS in the context of GKRS-treatments in functional brain areas.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Philippa Johnstone (Department of Oncology, Royal Berkshire, NHS Foundation Trust) for her valuable assistance in the format and editing of this paper.
References
1. Bailey PD, Zacà D, Basha MM, Agarwal S, Gujar SK, Sair HI. Presurgical fMRI and DTI for the Prediction of Perioperative Motor and Language Deficits in Primary or Metastatic Brain Lesions. J Neuroimaging. 2015. 25: 776-84
2. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985. 1: 1106-7
3. Bowden G, Niranjan A, Laing E, Pathak S, Flickinger J, Lunsford LD. Integration of magnetoencephalography-generated functional brain maps into dose planning during arteriovenous malformation radiosurgery. Stereotact Funct Neurosurg. 2014. 92: 103-8
4. Bulubas L, Sabih J, Wohlschlaeger A, Sollmann N, Hauck T, Ille S. Motor areas of the frontal cortex in patients with motor eloquent brain lesions. J Neurosurg. 2016. 125: 1431-42
5. Coburger J, Musahl C, Henkes H, Horvath-Rizea D, Bittl M, Weissbach C. Comparison of navigated transcranial magnetic stimulation and functional magnetic resonance imaging for preoperative mapping in rolandic tumor surgery. Neurosurg Rev. 2013. 36: 65-75
6. Conti A, Pontoriero A, Ricciardi GK, Granata F, Vinci S, Angileri FF. Integration of functional neuroimaging in CyberKnife radiosurgery: Feasibility and dosimetric results. Neurosurg Focus. 2013. 34: E5-
7. Conti A, Raffa G, Granata F, Rizzo V, Germanò A, Tomasello F. Navigated transcranial magnetic stimulation for. “somatotopic” tractography of the corticospinal tract. Neurosurgery. 2014. 10: 542-54
8. Conway N, Wildschuetz N, Moser T, Bulubas L, Sollmann N, Tanigawa N. Cortical plasticity of motor-eloquent areas measured by navigated transcranial magnetic stimulation in patients with glioma. J Neurosurg. 2017. 127: 981-91
9. Diehl CD, Schwendner MJ, Sollmann N, Oechsner M, Meyer B, Combs SE. Application of presurgical navigated transcranial magnetic stimulation motor mapping for adjuvant radiotherapy planning in patients with high-grade gliomas. Radiother Oncol. 2019. 138: 30-7
10. Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: Results and toxicity. Radiother Oncol. 2006. 81: 18-24
11. Espadaler J, Rogić M, Deletis V, Leon A, Quijada C, Conesa G. Representation of cricothyroid muscles at the primary motor cortex (M1) in healthy subjects, mapped by navigated transcranial magnetic stimulation (nTMS). Clin Neurophysiol. 2012. 123: 2205-11
12. Frey D, Schilt S, Strack V, Zdunczyk A, Rösler J, Niraula B. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. 2014. 16: 1365-72
13. Gavin CG, Ian Sabin H. Stereotactic diffusion tensor imaging tractography for Gamma Knife radiosurgery. J Neurosurg. 2016. 125: 139-46
14. Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010. 66: 113-20
15. Gupta SS. fMRI for mapping language networks in neurosurgical cases. Indian J Radiol Imaging. 2014. 24: 37-43
16. Hendrix P, Senger S, Griessenauer CJ, Simgen A, Schwerdtfeger K, Oertel J. Preoperative navigated transcranial magnetic stimulation in patients with motor eloquent lesions with emphasis on metastasis. Clin Anat. 2016. 29: 925-31
17. Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 2009. 74: 1543-8
18. Inoue HK, Seto K, Nozaki A, Torikai K, Suzuki Y, Saitoh J. Three-fraction cyberknife radiotherapy for brain metastases in critical areas: Referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J Radiat Res. 2013. 54: 727-35
19. Inuggi A, Filippi M, Chieffo R, Agosta F, Rocca MA, González-Rosa JJ. Motor area localization using fMRI-constrained cortical current density reconstruction of movement-related cortical potentials, a comparison with fMRI and TMS mapping. Brain Res. 2010. 1308: 68-78
20. Kato N, Schilt S, Schneider H, Frey D, Kufeld M, Vajkoczy P. Functional brain mapping of patients with arteriovenous malformations using navigated transcranial magnetic stimulation: First experience in ten patients. Acta Neurochir (Wien). 2014. 156: 885-95
21. Kawashima A, Krieg SM, Faust K, Schneider H, Vajkoczy P, Picht T. Plastic reshaping of cortical language areas evaluated by navigated transcranial magnetic stimulation in a surgical case of glioblastoma multiforme. Clin Neurol Neurosurg. 2013. 115: 2226-9
22. Kim JW, Park HR, Lee JM, Kim JW, Chung HT, Kim DG. Fractionated stereotactic gamma knife radiosurgery for large brain metastases: A retrospective, single center study. PLoS One. 2016. 11: e0163304-
23. Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014. 190: 521-32
24. Koga T, Maruyama K, Kamada K, Ota T, Shin M, Itoh D. Outcomes of diffusion tensor tractography-integrated stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012. 82: 799-802
25. Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg. 2012. 116: 994-1001
26. Krieg SM, Sollmann N, Tanigawa N, Foerschler A, Meyer B, Ringel F. Cortical distribution of speech and language errors investigated by visual object naming and navigated transcranial magnetic stimulation. Brain Struct Funct. 2015. 221: 2259-86
27. Krieg SM, Lioumis P, Mäkelä JP, Wilenius J, Karhu J, Hannula H. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir (Wien). 2017. 159: 1187-95
28. Kuchcinski G, Mellerio C, Pallud J, Dezamis E, Turc G, Rigaux-Viodé O. Three-tesla functional MR language mapping: Comparison with direct cortical stimulation in gliomas. Neurology. 2015. 84: 560-8
29. Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: The current evidence. Cancer Treat Rev. 2014. 40: 48-59
30. Mäkelä JP, Vitikainen AM, Lioumis P, Paetau R, Ahtola E, Kuusela L. Functional plasticity of the motor cortical structures demonstrated by navigated TMS in two patients with epilepsy. Brain Stimul. 2013. 6: 286-91
31. Maurer S, Giglhuber K, Sollmann N, Kelm A, Ille S, Hauck T. Non-invasive mapping of face processing by navigated transcranial magnetic stimulation. Front Hum Neurosci. 2017. 11: 4-
32. Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: A comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016. 95: 1142-8
33. Mohammadi AM, Schroeder JL, Angelov L, Chao ST, Murphy ES, Yu JS. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg. 2017. 126: 735-43
34. Negwer C, Sollmann N, Ille S, Hauck T, Maurer S, Kirschke JS. Language pathway tracking: Comparing nTMS-based DTI fiber tracking with a cubic ROIs-based protocol. J Neurosurg. 2017. 126: 1006-14
35. Niranjan A, Lunsford LD. Gamma knife radiosurgery for 5 to 10 brain metastases: A good option for upfront treatment. Oncology (Williston Park). 2016. 30: 314-317
36. Pantelis E, Papadakis N, Verigos K, Stathochristopoulou I, Antypas C, Lekas L. Integration of functional MRI and white matter tractography in stereotactic radiosurgery clinical practice. Int J Radiat Oncol Biol Phys. 2010. 78: 257-67
37. Picht T, Mularski S, Kuehn B, Vajkoczy P, Kombos T, Suess O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery. 2009. 65: 93-8
38. Picht T, Schilt S, Frey D, Vajkoczy P, Kufeld M. Integration of navigated brain stimulation data into radiosurgical planning: Potential benefits and dangers. Acta Neurochir (Wien). 2014. 156: 1125-33
39. Picht T, Frey D, Thieme S, Kliesch S, Vajkoczy P. Presurgical navigated TMS motor cortex mapping improves outcome in glioblastoma surgery: A controlled observational study. J Neurooncol. 2016. 126: 535-43
40. Raffa G, Bährend I, Schneider H, Faust K, Germanò A, Vajkoczy P. A Novel Technique for Region and Linguistic Specific nTMS-based DTI Fiber Tracking of Language Pathways in Brain Tumor Patients. Front Neurosci. 2016. 10: 552-
41. Raffa G, Conti A, Scibilia A, Sindorio C, Quattropani MC, Visocchi M. Functional Reconstruction of Motor and Language Pathways Based on Navigated Transcranial Magnetic Stimulation and DTI Fiber Tracking for the Preoperative Planning of Low Grade Glioma Surgery: A New Tool for Preservation and Restoration of Eloquent Networks. Acta Neurochir Suppl. 2017. 124: 251-61
42. Raffa G, Conti A, Scibilia A, Cardali SM, Esposito F, Angileri FF. The Impact of Diffusion Tensor Imaging Fiber Tracking of the Corticospinal Tract Based on Navigated Transcranial Magnetic Stimulation on Surgery of Motor-Eloquent Brain Lesions. Neurosurgery. 2018. 83: 768-82
43. Ruohonen J, Karhu J. Navigated transcranial magnetic stimulation. Neurophysiol Clin. 2010. 40: 7-17
44. Säisänen L, Könönen M, Julkunen P, Määttä S, Vanninen R, Immonen A. Non-invasive preoperative localization of primary motor cortex in epilepsy surgery by navigated transcranial magnetic stimulation. Epilepsy Res. 2010. 92: 134-44
45. Schmidt S, Fleischmann R, Bathe-Peters R, Irlbacher K, Brandt SA. Evolution of premotor cortical excitability after cathodal inhibition of the primary motor cortex: A sham-controlled serial navigated TMS study. PLoS One. 2013. 8: e57425-
46. Schwendner MJ, Sollmann N, Diehl CD, Oechsner M, Meyer B, Krieg SM. The Role of Navigated Transcranial Magnetic Stimulation Motor Mapping in Adjuvant Radiotherapy Planning in Patients With Supratentorial Brain Metastases. Front Oncol. 2018. 8: 424-
47. Shibamoto Y, Miyakawa A, Otsuka S, Iwata H. Radiobiology of hypofractionated stereotactic radiotherapy: What are the optimal fractionation schedules?. J Radiat Res. 2016. 57: i76-82
48. Sneed PK, Mendez J, Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J Neurosurg. 2015. 123: 373-86
49. Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016. 7: 12318-30
50. Sollmann N, Negwer C, Ille S, Maurer S, Hauck T, Kirschke JS. Feasibility of nTMS-based DTI fiber tracking of language pathways in neurosurgical patients using a fractional anisotropy threshold. J Neurosci Methods. 2016. 267: 45-54
51. Sollmann N, Laub T, Kelm A, Albers L, Kirschke JS, Combs SE. Predicting brain tumor regrowth in relation to motor areas by functional brain mapping. Neurooncol Pract. 2018. 5: 82-95
52. Stancanello J, Cavedon C, Francescon P, Causin F, Avanzo M, Colombo F. BOLD fMRI integration into radiosurgery treatment planning of cerebral vascular malformations. Med Phys. 2007. 34: 1176-84
53. Takahashi S, Jussen D, Vajkoczy P, Picht T. Plastic relocation of motor cortex in a patient with LGG (low grade glioma) confirmed by NBS (navigated brain stimulation). Acta Neurochir (Wien). 2012. 154: 2003-8
54. Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS. Preoperative multimodal motor mapping: A comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg. 2012. 117: 354-62
55. Tokarev AS, Rak VA, Sinkin MV, Evdokimova OL, Stepanov VN, Koynash GV. Appliance of Navigated Transcranial Magnetic Stimulation in Radiosurgery for Brain Metastases. J Clin Neurophysiol. 2019. p.
56. Tyndall AJ, Reinhardt J, Tronnier V, Mariani L, Stippich C. Presurgical motor, somatosensory and language fMRI: Technical feasibility and limitations in 491 patients over 13 years. Eur Radiol. 2017. 27: 267-78
57. Weiss Lucas C, Tursunova I, Neuschmelting V, Nettekoven C, Oros-Peusquens AM, Stoffels G. Functional MRI vs. navigated TMS to optimize M1 seed volume delineation for DTI tractography. A prospective study in patients with brain tumours adjacent to the corticospinal tract. Neuroimage Clin. 2017. 13: 297-309
58. Williams BJ, Suki D, Fox BD, Pelloski CE, Maldaun MV, Sawaya RE. Stereotactic radiosurgery for metastatic brain tumors: A comprehensive review of complications. J Neurosurg. 2009. 111: 439-48